Articular Cartilage
Articular cartilage is a tissue that is a component of synovial joints that can resist, absorb, dissipate, and transmit intermittent compression. This is in contrast to bone which is better at resisting static compression. The cartilage retains its form and remains available for the next phase of compression. It also provides a smooth surface over which a joint can glide.
Articular cartilage is on average 2 to 4mm thick (retropatellar up to 7 to 8mm), and it doesn't have blood vessels, nerves, or lymphatics.
Zonal Regions
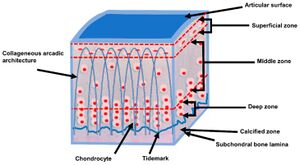
There are different classification systems to describe the four layers within articular cartilage. At the bottom of the deep layer is the tidemark. The tidemark is the boundary between uncalcified matrix above and calcified matrix below.
- Numerical system: identifies layers from I to IV from superficial to deep.
- Topographical system: identifies layers as superficial, middle, deep, and calcified layers. The calcified layer has received a deposit of calcium and is trying to transform into bone like the subchondral bone underlying it.
- Cellular orientation system: identifies layers as tangential in the superficial layer because they run tangential to the surface, transitional in the middle layer because they are clumped and scattered, and radial in the deep layer because they are arranged in radial columns. The fourth layer is again called calcified.
| Zone | Name | Description | Functional Behaviour |
|---|---|---|---|
| Zone I | superficial (tangential) zone | 10% to 20% of AC thickness. The collagen fibres of this zone are packed tightly and aligned parallel to the articular surface. The chondrocytes in the superficial zone are flatter and smaller and generally have a greater density than that of the cells deeper in the matrix. It protects deeper layers from shear stresses. The zona superficialis has most of the water and very little proteoglycans. In general, the cells are not very active, which means that there is little wear and tear. The zone can also be used as a barrier against large molecules, for example antibodies | Responsible for the behaviour of the cartilage under stress. It deforms more strongly and is therefore less rigid than the underlying zones. If this zone is disturbed tissue permeability increases, leading to greater fluid exchange of the cartilage with its surroundings and during compression, this leads to greater mechanical stress on the macromolecular network. The cells here also produce lubricin. |
| Zone II | middle (transitional) zone | 40% to 60% of the total AC volume, and it contains proteoglycans and thicker collagen fibrils. In this layer, the collagen is organized obliquely. Chondrocytes are spherical and at low density | First line of resistance against compressive forces. The upper aspect produces lubricin. |
| Zone III | deep (radial) zone | Approximately 30% of the AC volume. The deep zone contains the largest diameter collagen fibrils in a radial disposition, the highest proteoglycan content, and the lowest water concentration. The chondrocytes are typically arranged in a columnar orientation, parallel to the collagen fibers and perpendicular to the joint line. | Responsible for providing the greatest resistance to compressive forces, given that collagen fibrils are arranged perpendicular to the articular surface. This creates a arcade formation. These arcades are to be created by the attempt to transfer the initial fibril network to a higher order, whereby the arcadic structure supports the overlying load. |
| Interface | tide mark | Distinguishes the deep zone from the calcified cartilage. This irregularly salty layer lies between the Zonae radiata and calcificata and separates the lime-poor from the lime-rich cartilage. This has been described as the mineralization front of the cartilage. The layer is thought to arise by causing the collagen bundles to twist before going deeper to penetrate the zona calcificata and the bone tissue | Consists of a band of fibrils attached to the collagen fibers which are anchored in the lime-poor layer, and thus prevent them from tearing off cartilage from bone |
| Zone IV | calcified zone | Plays an integral role in securing the cartilage to bone by anchoring the collagen fibrils of the deep zone to subchondral bone. In this zone, the cell population is scarce and chondrocytes are hypertrophic | Numerous protrusions, hollows, and interlacing, which gives an excellent resistance to shear forces to prevent the cartilage detaching from the underlying bone |
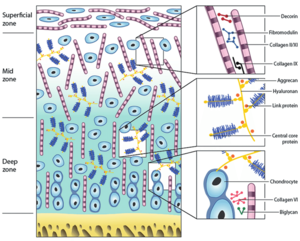
The cellular arrangement reflects the function of each layer. The superficial layer has cells arranged tangentially because it is subjected to both compression and friction. In cross-section these superficial cells are discoid. In comparison, the middle layer cells are spherical in cross-section.
The collagen within the matrix has a certain arrangement. It passes upwards through the matrix and surrounds cells in an arcadic manner. This disposition retains the matrix in discrete regions. In the superficial layer the final arch contains a layer of collagen that run parallel to the cells in the direction of greatest insult.
Cellular density is highest at the superficial layer, with reducing density down to the deep layer.
Components of Articular Cartilage
The critical component of cartilage is water. The proteoglycans include aggrecan, dermatosulphate proteoglycans, and fibromodulin. The main collagen type found is type II. Non-collagenous proteins include structural glycoproteins and matrix metalloproteinases.
| Component of Articular Cartilage | Percentage | |
|---|---|---|
| Water | 70 - 80% | |
| Chondrocytes | 1 - 10% | |
| Collagen | 12 - 14% | |
| - Type II | 10 - 12% | |
| - Type IX | ~1% | |
| - Type XI | ~1% | |
| Proteoglycans | 7 - 9% | |
| - Hyaluronic acid—proteoglycans—aggregates | 6 - 8% | |
| - Other proteoglycans | ~1% | |
| Mineralic materials | <4% | |
| Matrix metalloproteinases | <1% |
Water
The matrix is composed of water, proteoglycans, collagen, and non-collagenous proteins. Water is found in the joint and water alone can withstand intermittent compression. Water is compressible but with compression it has the undesired effect of flowing and dissipating. Water is therefore held in place thermodynamically and electrostatically by proteoglycans. Proteoglycans are retained in turn by collagen. The collagen is responsible for maintaining the articular cartilage in a form to resist the next phase of compression.
In other words water is retained by proteoglycans, and proteoglycans are bound by collagen. Proteoglycans and collagen are produced by cells. Non-collagenous proteins are also produced that function to hold the components together and regulate them. Chondrocytes are therefore required to afford the cartilage its mechanical properties.
Chondrocytes
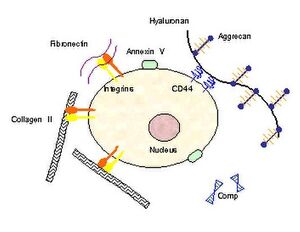
There is a zone of matrix that immediately surrounds the cells known as peri-cellular matrix. Then there is a region of matrix surrounding the cell but more displaced known as the territorial matrix. The matrix in between cells or clusters of cells is known as interterritorial matrix. Collagen is the dominant constituent in the interterritorial matrix. In the pericellular matrix the dominant constituents are proteoglycans and hyaluronic acid.
Cyclic compressive loading is important for nutrition to the chondrocytes and waste removal because cartilage is an avascular tissue. Waste is removed with compression, and nutrients diffuse in from synovial fluid with removal of compression. Excessive loading may negatively influence the cells resulting in cell apoptosis and destruction of the matrix.[2]
Chondrocytes have mechanoreceptors on their surface. These receptors sense and transform the mechanical signal into biochemical signals that results in the synthesis and degradation of the components of the cartilaginous extracellular matrix. The processes include the synthesis of collagens, proteoglycans, proteases, protease inhibitors, transcription factors, cytokines, and growth factors. The balance between the anabolic and catabolic processes is influenced by the type of loading that the cartilage experiences. High strain rates cause tissue damage, degradation, decreased matrix production, and apoptosis. While decreased mechanical loading leads to a loss of matrix production. Low frequency oscillatory loads are beneficial, while static loads are detrimental.
Aggrecan and Aggregated Proteoglycans
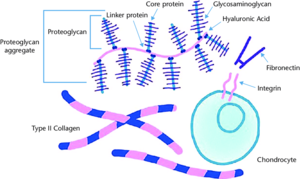
There are three macroclasses of proteins in articular cartilage: collagens (mostly type II collagen); proteoglycans (mostly aggrecan); and other non-collagenous proteins (link protein, fibronectin, matrix protein) and smaller proteoglycans (biglycan, decorin, fibromodulin).[1]
Aggrecan being the major proteoglycan in articular cartilage contains a core protein with numerous covalently attached sulfated glycosaminoglycan (GAG) chains. These GAGs are in the form of chondroitin sulfate and keratan sulfate. A greater proteoglycan concentration provides greater resistance to shear forces. The highest density is found in the middle zone.
Aggrecan doesn't exist in isolation but rather in the form of proteoglycan aggregates. Each aggregate contains a central filament of hyaluronic acid with multiple aggrecan molecules attached non-covalently via one terminus of their core proteins. A link protein stabilises the interaction between the aggrecan and hyaluronic acid.[2]
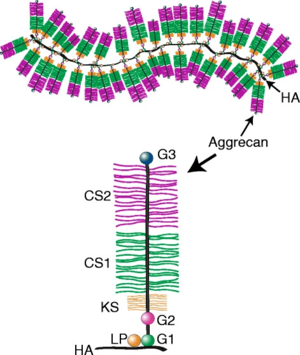
The core protein of aggrecan contains three disulfide-bonded globular regions which are termed G1, G2, and G3. There are what are called "extended domains" between G1 and G2 called E1 (also called interglobular domain); and between G2 and G3 called E2. [2]
The proximal G1 region resembles an immunoglobulin and is called the immunoglobulin fold (Ig fold). This end is responsible for linking to hyaluronic acid and held to it by a similarly shaped link protein.
The function of G2 is unknown but is structurally similar to G1. There is a short interglobular region (sometimes called E1) between G1 and G2, and this region is a prominent site of proteolysis.[2]
The interglobular region between G2 and G3 is longer (sometimes called E2). It is here that the water trapping glycosaminoglycans chondroitin sulfate and keratan sulfate are attached to the protein core. The term water trapping is used because there the binding process is by both electrostatic and thermodynamic forces. Most keratan sulfate is found in the proximal end of E2, which is called the KS region. All of the chondroitin sulfate is found in the terminal three quarters of E2, of which there are the CS1 and CS2 regions. This area also contains some keratan sulfate.[2]
The G3 region is involved in normal transit of the aggrecan within the chondrocyte and its secretion into the matrix. It also has a role in anchoring aggrecan within the tissue through binding to other proteins such as fibulins and tenascins. G3 is absent in many mature aggrecan molecules due to its protelytic cleavage,[2] but mutations that abolish binding of G3 to fibulins and tenascins leads to familial osteochondritis dissecans.[3]
Dermatosulphate Proteoglycans
Interspersed throughout the matrix are a variety of other proteoglycans. The dermatosulphate proteoglycans are biglycan and decorin. Bogduk includes fibromodulin in this group. These are proteoglycans with a protein core from which stem glycosaminoglycans.
- Biglycan has two glycosaminoglycan chains and it acts as an inhibitor of fibrinogen. Repair involves activation of fibrinogen and deposition of fibrin.
- Decorin can interact with collagen and inhibit fibrillogenesis. Fibrillogenesis is the manufacture of thicker collagen fibres. Decorin can also bind to chondronectin and growth factors such as TGF. It has a wide range of effects including cell growth, differentiation, proliferation, adhesion, spread and migration, and has pro-inflammatory and anti-fibrosis effects.[4]
- Fibromodulin has a protein core and small glycosaminoglycan chain. Fibromodulin sits on the surface of collagen fibres and regulates their assembly. It inhibits the deposition of proto-collagen fibres onto existing collagen fibres.
Collagens
There are a variety of collagens in cartilage that come from various chemical classes of collagen.
Class 1 collagens are the fibril forming collagens, those that can make strings. The main example in articular cartilage is type II collagen which comprises some 90% to 95% of the collagen and is responsible for the tensile strength of the tissue. Type XI collagen is responsible for controlling fibril diameter. Type VI is found in the territorial matrix.
Class 3 collagens are those that form short helices. Type IX collagen is responsible for bridging between itself and type II. Type X is found in zone IV.
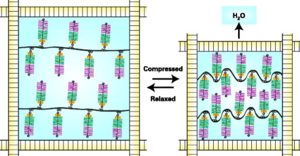
Collagen maintains the volume and shape of the matrix because by winding around and entrapping the proteoglycan aggregates it restricts the swelling of the proteoglycans with water imbibement to about 20% of their maximum.
In their relaxed state the aggregates swell as the anionic chondroitin sulfate and keratan sulfate GAG chains draw in water. An equilibrium is reached whereby the swelling is in balance with the tensile forces of the collagen fibrils. With compression, water is displaced, and the GAG chains are brought closer together. This increases their swelling potential and balances the applied load. The increased swelling potential is dissipated when the load is removed, the aggrecan re-swells, and the original equilibrium is restored.[2]
The most important property of collagen is its tensile strength. Collagen buckles when it is compressed. The tensile strength properties of collagen is complementary to proteoglycans which ensure compressive strength, elasticity, and the lifespan of articular cartilage. [1]
For optimal cartilage function and the ability to reach this equilibrium, the cartilage requires a high concentration of aggrecan, a high degree of aggrecan sulfation, and the capacity to form large aggregates. All three of these properties are impaired in osteoarthritis.[2]
Non-collagenous Proteins
The structural glycoproteins are molecules found on the surface of cells referred to as adhesion molecules. They bind chondroblasts to type II collagen. We see an interaction between the cells that produce the matrix and the matrix components itself. The best known structural glycoproteins are chondronectin and anchorin.
Cartilage oligomerix protein (COMP) is also known as thrombospondin 5. It's exact function remains unclear but it appears to be an important regulator of extracellular matrix assembly and stabilisation of the matrix through interacting between collagen fibrils and matrix components. It has been studied as both a diagnostic and prognostic indicator of osteoarthritis; it is significantly upregulated in early osteoarthritis even long before diagnosis.[5]
MMPs
Some of the best known proteolytic enzymes are the matrix metalloproteinases (MMPs), and as indicated by their name occur in the matrix. Metallo refers that these enzymes require zinc as a component that allows them to act on the proteins. Calcium is involved in this interaction.
MMPs are secreted in an inactive form, with proteolytic loss of the propeptide leading to activation in the matrix. For example MMP-1, -2, and -3 are produced as pro-collagenase, pro-gelatinase, and pro-stromelysin, respectively. In contrast, the membrane type MMPs (MT-MMPs) and stomelysin-3 (MMP-11) are secreted as active enzymes. Most MMPs are produced by different connective tissue cells after stimulation by a variety of mediators.[6]
There are some 29 MMPs, and they are divided into four main groups:[3]
- Collagenases: (e.g. MMP-1, -8, -13). These enzymes cleave fibrillar collagens at a single site to produce fragments of three quarters and one quarter of the original size. MMP-13 has the broadest substrate specificity. They have strong activity for cleavage of type II collagen, but other molecules such as types IV and IX collagen, perlecan, osteonectin, and proteoglycan. MMP-1 and MMP-13 are synthesised by macrophages, fibroblasts, and chondrocytes upon stimulation by inflammatory mediators. MMP-8 is predominantly released by neutrophils upon stimulation but also by chondrocytes. All three are present in diseased cartilage.
- Gelatinases: (MMP-2 and -9) These act on breakdown products of type IV and V collagen and elastin. MMP-2 is the most widely expressed.
- Stromelysins: (e.g. MMP-3). These acts on a wide array of extracellular molecules including various collagens, fibronectin, laminin, elastin, and various proteoglycans.
- MT-MMPs (e.g. MMP-14): MMP-14 can initiate activation cascades on the cell surface that activate procollagenases.
ADAMTS and ADAMS
Relatives of the MMPs are the ADAMTS enzymes (short for A Disintegrin And Metalloproteinase with Thrombospondin Motifs) and ADAMs (A Disintegrin And Metalloproteinases). These enzymes are also dependent on zinc. These enzymes were more recently discovered. There are 20 ADAMTS and 33 ADAMS enzymes, with a large number of them having effects on cartilage.
ADAMTS enzymes are involved in both the synthesis and degradation of the extracellular matrix. ADAMTS enzymes include:[3]
- Procollagen propeptidases (ADAMTS-2, -3, and -14) that are involved in the maturation of triple helical collagen fibrils
- Aggrecanases (ADAMTS-1, -4, -5, -9, and -15) that are involved in aggrecan cleavage along with MMPs. ADAMTS5 is about 1,000 times more potent than ADAMTS4.[7]
ADAMTS enzymes are also involved in the cleavage of oligomerix matrix protein, have roles in cartilage metabolism, as well as in chondrocyte differentiation and proliferation. The role that the various ADAMTS and ADAMS enzymes play in joint health and disease is still being studied.[7]
ADAMs are usually membrane-anchored proteinases. It is thought that they play a role in signalling pathways in chondrocytes to fine-tune tissue homeostasis.[8]
Serine Proteinases
Serine proteinases are understudied despite there being almost as many of these as there are MMPs. They are thought to be involved in regulation of cell signalling, modulation of the activity of growth factors, promotion of inflammatory processes, modulation of chemokines, and as a protective feedback mechanism in maintaining cartilage homeostasis.[3]
Matrix Synthesis and Degradation
The chondrocytes slowly but constantly act to have effects in both the synthesis and degradation of the extracellular matrix. Degradation is an important aspect as it allows for old components to be removed to making way for refreshed components. In the normal state there is a balance between synthesis and degradation. If the balance tips to favouring degradation, then degenerative changes occur.
Synthesis is promoted by various growth factors including transforming growth factor (TGF), basic fibroblast growth factor (bFGF), and insulin like growth factor (IGF). Degradation occurs through the action of metalloproteases. MMP-13 plays a central role in degrading type II collagen, while ADAMT-4 and -5 are primary agents that degrade aggrecan. Tumour necrosis factor α interleukin-1 (IL-1) promote the action of metalloproteinases, but degradation also occurs through superoxide (O2−) and nitric oxide.
Synthesis
The chondroblast produces the components of the extracellular matrix. The endoplasmic reticulum within the chondroblast synthesises the various collagens and other proteins of the matrix such as fibromodulin and decorin. At the base of the golgi apparatus there is some endoplasmic reticulum that forms the protein component of the proteoglycans. The carbohydrate component is added to the protein core by the golgi apparatus. Anchorin is an adhesion molecule that sits on the surface of the chondroblast.
The chondroblast can be stimulated by a variety of factors to produce collagen and proteoglycans.[3]
- Fibroblast growth factor (FGF) is an extrinsic regulator that comes from outside the cell and stimulates the nucleus of the chondroblast to produce the protein and proteoglycan components of the matrix.
- Insulin like growth factor (IGF) also comes from the outside, and activates the nucleus by a second messenger to produce the components of the matrix.
- Transforming growth factor (TGF) is produced by the cell itself, and is bound to collagen through decorin. While TGF is buried within the proteoglycans surrounding collagen it can't escape and has no influence. If the proteoglycans are removed and the collagen is exposed, so is TGF, and floats back to the cell where it enhances the effect of FGF through a positive feedback loop. This activates the cell to produce materials, with a preferential effect for the production of proteoglycans, which closes the loop by enclosing collagen and blocking the effect of TGF.
- Bone morphogenic proteins (BMPs): these have direct effects on chondrocytes and are involved in promoting matrix deposition in osteoarthritis (e.g. osteophytosis).
Degradation
Cartilage produce many enzymes that can destroy the matrix. The complete collection of proteases is called the degradome and includes around 588 different proteinases. These include the Matrix Metalloproteinases (MMPs), the A Disintegrin And Metalloproteinase Domain with Thrombospondin Motifs (ADAMTS) family, and the serine proteinases. With the primary structural components of cartilage matrix being collagen and aggrecan, MMP-13 and ADAMTS-4 and -5 play a central role in cartilage degradation.
The ADAMTS are important in the initial degradation of cartilage matrix. ADAMTS-4 and ADAMTS-5 can cleave the interglobular domain between G1 and G2, leaving a shortened aggrecan with decreased numbers of fixed charged groups. This is thought to be a key step in the pathologic loss of aggrecan. They can also cleave several sites close to the G3 domain. Following cleavage between G1 and G2, the G1 domain remains bound to hyaluronic acid can then be cleaved by MMPs. Also of note, the combined action of the ADAMTs aggrecanases and MMPs results in the release of a soluble bioactive peptide that promotes further cartilage breakdown.[3]
MMP-1 and MMP-13 are both relatively specific for action on type II collagen. They act on type II collagen up towards one end. Once the collagen molecule is split, gelatinase acts on the products. MMP-13 is thought to also be involved in matrix turnover in healthy cartilage. MMP-13 (Collagenase-3) is considered to be the major catabolic effector in osteoarthritis, while MMP-1 is thought to be the primary collagenase in rheumatoid arthritis. MMP-13 It has been extensively studied and selective MMP-13 inhibitors for treating osteoarthritis are being developed.[7] The role of MMP-1 in osteoarthritis may vary depending on the joint.[9]
The other components of the matrix are acted on by MMP-3 (stromelysin). MMP-3 is the most highly expressed MMP gene in normal cartilage and remains comparatively high in osteoarthritis. MMP-3 can also cleave aggrecan molecules between G1 and G2. Other processes operate to liberate the link protein and head end of the aggrecan molecule. These products leak into the synovial fluid and removed via transportation through lymph. MMP-3 can act on other collagens within the matrix such as type XI and IX and the terminal ends of type II collagen. MMP-3 can also act on smaller collagens found around the cell such as type XI, and other proteins such as fibronectin. MMP-3 is a good general debriding enzyme. In osteoarthritis there appears to be an excessive degradation of the matrix.
A feature of aging cartilage is the accumulation of hyaluronic acid chains that bear the old remnant head end of the aggrecan molecule, that isn't removed, and this remnant prevents the binding of fresh aggrecan molecules. This contributes to the compromise of articular cartilage with age.
In healthy articular cartilage there the extracellular matrix is slowly but continuously refreshed. Old collagen is removed and replaced by new collagen; old proteoglycans are removed and replaced by new proteoglycans. There is a balance between synthesis and degradation of the matrix.
Tissue inhibitors of metalloproteinases (TIMP) can control the activation of the MMPs and ADAMTS enzymes preventing uninhibited action of these enzymes. With reduced activity of TIMPs there may be excessive degradation for the amount of synthesis occurring.
Cytokines that have an effect on the chondroblast and promote degradation include interleukin (IL), tumour necrosis factor α (TNF), and interferon (IFN). These mediators have two sorts of effects. There is a lesser effect of stimulation of the production of degradative enzymes. The dominant effect is the inhibition of the synthesis of the proteins, collagens, and proteoglycans. Superoxide radicals and nitric oxide also promote degradation. Inflammatory effects do not explain all of the features of the genesis of osteoarthritis.
See Also
References
- ↑ 1.0 1.1 1.2 1.3 Eschweiler J, Horn N, Rath B, Betsch M, Baroncini A, Tingart M, Migliorini F. The Biomechanics of Cartilage-An Overview. Life (Basel). 2021 Apr 1;11(4):302. doi: 10.3390/life11040302. PMID: 33915881; PMCID: PMC8065530.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Roughley, P.J., Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J EXP ORTOP 1, 8 (2014). https://doi.org/10.1186/s40634-014-0008-7
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Hochberg, Marc C., et al. Rheumatology. Philadelphia, PA: Elsevier, 2019.
- ↑ Zhang, Wen et al. “Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment.” Oncotarget vol. 9,4 5480-5491. 3 Jan. 2018, doi:10.18632/oncotarget.23869
- ↑ Tseng, Susan et al. “Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis.” Biomarker insights vol. 4 33-44. 17 Feb. 2009, doi:10.4137/bmi.s645
- ↑ Brandon J. Rose, David L. Kooyman, "A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology", Disease Markers, vol. 2016, Article ID 4895050, 7 pages, 2016. https://doi.org/10.1155/2016/4895050
- ↑ 7.0 7.1 7.2 Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the 'usual suspects'. Osteoarthritis Cartilage. 2017 Jul;25(7):1000-1009. doi: 10.1016/j.joca.2017.02.791. Epub 2017 Feb 13. PMID: 28216310; PMCID: PMC5473942.
- ↑ Yang, C-Y et al. “ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the 'usual suspects'.” Osteoarthritis and cartilage vol. 25,7 (2017): 1000-1009. doi:10.1016/j.joca.2017.02.791
- ↑ Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004 Jan;50(1):131-41. doi: 10.1002/art.11433. PMID: 14730609.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,