Chronic Inflammatory Demyelinating Polyneuropathy: Difference between revisions
(Created page with "{{stub}} {{condition |image= |name= |taxonomy= |synonym= |definition= |epidemiology=0.7-1.6 cases per 100,000 per year, median age 58 |causes= |pathophysiology=Autoimmune demy...") |
No edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{partial}} | ||
{{condition | {{condition | ||
|image= | |image= | ||
| Line 13: | Line 13: | ||
|secondaryprevention= | |secondaryprevention= | ||
|riskfactors=Diabetes, anti TNF-alpha medication | |riskfactors=Diabetes, anti TNF-alpha medication | ||
|history= | |history=Proximal and distal weakness, paraesthesias, numbness, fatigue, difficulty with fine motor control | ||
|examination= | |examination=Weakness, areflexia without wasting, joint position and vibration sense loss, foot drop, sensory ataxia | ||
|diagnosis=[[:File:CIDP diagnostic criteria.jpg| | |diagnosis=[[:File:CIDP diagnostic criteria.jpg|Diagnostic criteria]] | ||
|tests= | |tests=Laboratory tests, electrophysiology studies | ||
|ddx= | |ddx= | ||
|treatment=Immunoglobulin and plasma exchange | |treatment=Immunoglobulin and plasma exchange | ||
| Line 22: | Line 22: | ||
}} | }} | ||
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) is an acquired, immune-mediated demyelinating polyneuropathy that affects peripheral nerves and nerve roots. It has an extremely variable presentation and is often difficult to diagnose. It is important to be aware of as an MSK physician because pain can be one of the manifestations. | |||
== Epidemiology == | |||
The prevalence has been estimated to be between 0.67-10.3 per 100,000 people. There is an increasing incidence and prevalence with age. It is two times more common in men than women. There are no clear predisposing risk factors.<ref name=":1">{{Cite journal|last=Bunschoten|first=Carina|last2=Jacobs|first2=Bart C.|last3=Van den Bergh|first3=Peter Y. K.|last4=Cornblath|first4=David R.|last5=van Doorn|first5=Pieter A.|date=2019-08|title=Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy|url=https://pubmed.ncbi.nlm.nih.gov/31076244|journal=The Lancet. Neurology|volume=18|issue=8|pages=784–794|doi=10.1016/S1474-4422(19)30144-9|issn=1474-4465|pmid=31076244}}</ref> | |||
The common manifestations of CIDP are: | In neuromuscular disease referral centres, CIDP is determined to be the cause of around 20% of all undiagnosed neuropathies, and 10% of all patients referred.<ref name=":2">Case Files Neurology. 2017</ref> | ||
== Pathogenesis == | |||
The aetiology is immunologic through cell-mediated and antibody-mediated mechanisms. There is persistent multi-focal demyelination that has a predilection for the spinal roots, major plexuses, and proximal nerve trunks. Histologically there are patchy areas of demyelination and oedema with variable inflammatory infiltrates of macrophages and T cells. | |||
== Clinical Features == | |||
CIDP should be considered in any patient with progressive symmetrical or asymmetrical polyradiculopathy that is relapsing and remitting or that progresses for longer than 2 months.<ref name=":0">{{Cite journal|last=Ryan|first=Melody|last2=Ryan|first2=Stephen J.|date=2018-09|title=Chronic inflammatory demyelinating polyneuropathy: considerations for diagnosis, management, and population health|url=https://pubmed.ncbi.nlm.nih.gov/30312032|journal=The American Journal of Managed Care|volume=24|issue=17 Suppl|pages=S371–S379|issn=1936-2692|pmid=30312032}}</ref> | |||
The pace of onset is usually chronic, but can sometimes be more acute. There is a temporal continuum between the demyelinating form of Guillain-Barre on the one end (acute inflammatory demyelinating polyneuropathy - AIDP), and CIDP in those with AIDP that don't recover. Some patients will follow a relapsing course.<ref name=":1" /><ref name=":2" /> | |||
Patients usually complain of a generalised pattern of numbness and weakness in the upper and lower extremities along with spontaneous pain. Some patients may present with a progressive sensory ataxia, while in other motor symptoms are more dominant. | |||
{| class="wikitable" | |||
! The common manifestations of CIDP are: | |||
!Prevalence | |||
|- | |||
|Gradually worsening paraesthesia and numbness | |||
|72-89% | |||
|- | |||
|Muscle weakness in legs and arms | |||
|83-94% | |||
|- | |||
|Areflexia without wasting | |||
|86-94% | |||
|- | |||
|Facial palsy | |||
|4-15% | |||
|- | |||
|Preferential loss of joint position or vibration sense | |||
| | |||
|- | |||
|Foot drop and difficulty getting out of the chair | |||
| | |||
|- | |||
|Difficulty with fine motor control | |||
| | |||
|- | |||
|Sensory ataxia | |||
| | |||
|- | |||
|Fatigue | |||
| | |||
|- | |||
|Severe Pain | |||
|13-17% | |||
|} | |||
Notably motor and proprioceptive deficits predominate over pain and autonomic symptoms. Severe pain occurs in 13-17% of patients. | Notably motor and proprioceptive deficits predominate over pain and autonomic symptoms. Severe pain occurs in 13-17% of patients. | ||
The typical form of CIDP | The typical form of CIDP (>50%) is a sensory-motor phenotype. The symptoms are usually proximal rather than distal and roughly symmetrical. The acute form (18%) manifests with both proximal and distal symptoms. Atypical forms include a predominant distal distribution (distal acquired demyelinating symmetric - DADS), an asymmetric distribution (multifocal acquired demyelinating sensory and motor neuropathy - MADSAM), pure sensory, pure motor, and extremely rarely focal CIDP. | ||
== Investigations == | |||
The major tests are EMG/NCS, CSF examination, nerve biopsy, MRI, and blood tests. | |||
* CSF shows increased protein content and reduced cell count (called cyto-albuminologic dissociation). | |||
* Neurophysiology results may be non-specific and show mixed results because of the concomitant secondary axon degeneration on top of the demyelination. There is also a high prevalence of user error in the electrophysiology technique and interpretation. | |||
* Nerve biopsy is at risk of sampling error when a biopsy is taken proximal to the site of biopsy showing only nonspecific lesions. | |||
* MRI can provide supportive information such as hypertrophy and high signal in the nerve roots and plexi. | |||
* Skeletal surveys or scintigrophy: Can be done to detect plasmacytoma or myeloma. | |||
* Blood tests: tests are done to rule out other causes or associated conditions: FBC, HbA1c, creatinine, electrolytes, LFTs, TSH, B12/folate, protein electrophoresis, light chains. Additional tests to consider are HIV, neuroborreliosis, and ANA.<ref name=":1" /> | |||
== Diagnosis == | |||
Diagnosis is difficult and misdiagnosis is very common in general neurology practice. | |||
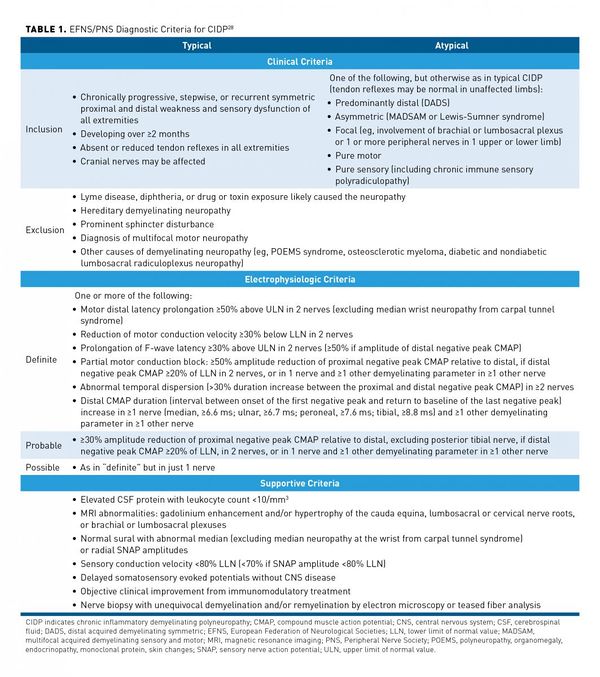
There are at least 15 different diagnostic criteria. See below for an example from the European Federation of Neurological Societies and the Peripheral Nerve Society. The criteria are highly specific (96.2%) but not sensitive (81.3%). Clinical history, physical examination, [[Nerve Conduction Studies|Electrophysiology]] studies, and laboratory tests form part of the criteria. Misdiagnosis is common, with misinterpretation of nerve conduction studies being a common reason. | |||
[[File:CIDP diagnostic criteria.jpg|600px]] | |||
== Differential Diagnosis == | |||
The list of differentials is extensive. | |||
== Treatment == | |||
Corticosteroids, intravenous immunoglobulin, plasma exchange, immunosuppresive drugs. | |||
== Resources == | |||
See Ryan et al for an open access review.<ref name=":0" /> | |||
==References== | ==References== | ||
<references/> | <references/> | ||
{{Reliable sources}} | {{Reliable sources}} | ||
[[Category: | [[Category:Polyneuropathies]] | ||
Latest revision as of 15:28, 11 March 2023
| Chronic Inflammatory Demyelinating Polyneuropathy | |
|---|---|
| Epidemiology | 0.7-1.6 cases per 100,000 per year, median age 58 |
| Pathophysiology | Autoimmune demyelination of large peripheral nerve fibres |
| Classification | Typical and atypical phenotypes |
| Risk Factors | Diabetes, anti TNF-alpha medication |
| History | Proximal and distal weakness, paraesthesias, numbness, fatigue, difficulty with fine motor control |
| Examination | Weakness, areflexia without wasting, joint position and vibration sense loss, foot drop, sensory ataxia |
| Diagnosis | Diagnostic criteria |
| Tests | Laboratory tests, electrophysiology studies |
| Treatment | Immunoglobulin and plasma exchange |
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) is an acquired, immune-mediated demyelinating polyneuropathy that affects peripheral nerves and nerve roots. It has an extremely variable presentation and is often difficult to diagnose. It is important to be aware of as an MSK physician because pain can be one of the manifestations.
Epidemiology
The prevalence has been estimated to be between 0.67-10.3 per 100,000 people. There is an increasing incidence and prevalence with age. It is two times more common in men than women. There are no clear predisposing risk factors.[1]
In neuromuscular disease referral centres, CIDP is determined to be the cause of around 20% of all undiagnosed neuropathies, and 10% of all patients referred.[2]
Pathogenesis
The aetiology is immunologic through cell-mediated and antibody-mediated mechanisms. There is persistent multi-focal demyelination that has a predilection for the spinal roots, major plexuses, and proximal nerve trunks. Histologically there are patchy areas of demyelination and oedema with variable inflammatory infiltrates of macrophages and T cells.
Clinical Features
CIDP should be considered in any patient with progressive symmetrical or asymmetrical polyradiculopathy that is relapsing and remitting or that progresses for longer than 2 months.[3]
The pace of onset is usually chronic, but can sometimes be more acute. There is a temporal continuum between the demyelinating form of Guillain-Barre on the one end (acute inflammatory demyelinating polyneuropathy - AIDP), and CIDP in those with AIDP that don't recover. Some patients will follow a relapsing course.[1][2]
Patients usually complain of a generalised pattern of numbness and weakness in the upper and lower extremities along with spontaneous pain. Some patients may present with a progressive sensory ataxia, while in other motor symptoms are more dominant.
| The common manifestations of CIDP are: | Prevalence |
|---|---|
| Gradually worsening paraesthesia and numbness | 72-89% |
| Muscle weakness in legs and arms | 83-94% |
| Areflexia without wasting | 86-94% |
| Facial palsy | 4-15% |
| Preferential loss of joint position or vibration sense | |
| Foot drop and difficulty getting out of the chair | |
| Difficulty with fine motor control | |
| Sensory ataxia | |
| Fatigue | |
| Severe Pain | 13-17% |
Notably motor and proprioceptive deficits predominate over pain and autonomic symptoms. Severe pain occurs in 13-17% of patients.
The typical form of CIDP (>50%) is a sensory-motor phenotype. The symptoms are usually proximal rather than distal and roughly symmetrical. The acute form (18%) manifests with both proximal and distal symptoms. Atypical forms include a predominant distal distribution (distal acquired demyelinating symmetric - DADS), an asymmetric distribution (multifocal acquired demyelinating sensory and motor neuropathy - MADSAM), pure sensory, pure motor, and extremely rarely focal CIDP.
Investigations
The major tests are EMG/NCS, CSF examination, nerve biopsy, MRI, and blood tests.
- CSF shows increased protein content and reduced cell count (called cyto-albuminologic dissociation).
- Neurophysiology results may be non-specific and show mixed results because of the concomitant secondary axon degeneration on top of the demyelination. There is also a high prevalence of user error in the electrophysiology technique and interpretation.
- Nerve biopsy is at risk of sampling error when a biopsy is taken proximal to the site of biopsy showing only nonspecific lesions.
- MRI can provide supportive information such as hypertrophy and high signal in the nerve roots and plexi.
- Skeletal surveys or scintigrophy: Can be done to detect plasmacytoma or myeloma.
- Blood tests: tests are done to rule out other causes or associated conditions: FBC, HbA1c, creatinine, electrolytes, LFTs, TSH, B12/folate, protein electrophoresis, light chains. Additional tests to consider are HIV, neuroborreliosis, and ANA.[1]
Diagnosis
Diagnosis is difficult and misdiagnosis is very common in general neurology practice.
There are at least 15 different diagnostic criteria. See below for an example from the European Federation of Neurological Societies and the Peripheral Nerve Society. The criteria are highly specific (96.2%) but not sensitive (81.3%). Clinical history, physical examination, Electrophysiology studies, and laboratory tests form part of the criteria. Misdiagnosis is common, with misinterpretation of nerve conduction studies being a common reason.
Differential Diagnosis
The list of differentials is extensive.
Treatment
Corticosteroids, intravenous immunoglobulin, plasma exchange, immunosuppresive drugs.
Resources
See Ryan et al for an open access review.[3]
References
- ↑ 1.0 1.1 1.2 Bunschoten, Carina; Jacobs, Bart C.; Van den Bergh, Peter Y. K.; Cornblath, David R.; van Doorn, Pieter A. (2019-08). "Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy". The Lancet. Neurology. 18 (8): 784–794. doi:10.1016/S1474-4422(19)30144-9. ISSN 1474-4465. PMID 31076244. Check date values in:
|date=(help) - ↑ 2.0 2.1 Case Files Neurology. 2017
- ↑ 3.0 3.1 Ryan, Melody; Ryan, Stephen J. (2018-09). "Chronic inflammatory demyelinating polyneuropathy: considerations for diagnosis, management, and population health". The American Journal of Managed Care. 24 (17 Suppl): S371–S379. ISSN 1936-2692. PMID 30312032. Check date values in:
|date=(help)
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,