Dermatomes: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 3: | Line 3: | ||
|Additional contributors= | |Additional contributors= | ||
|Peer reviewer=Lucy | |Peer reviewer=Lucy | ||
}} | |Review complete=Yes | ||
}}{{Peer reviewed}} | |||
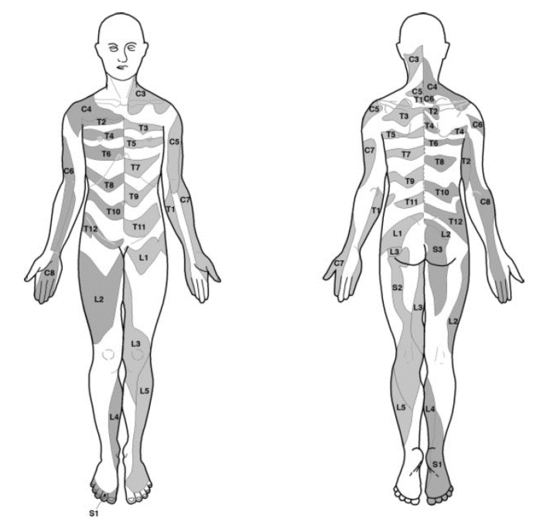
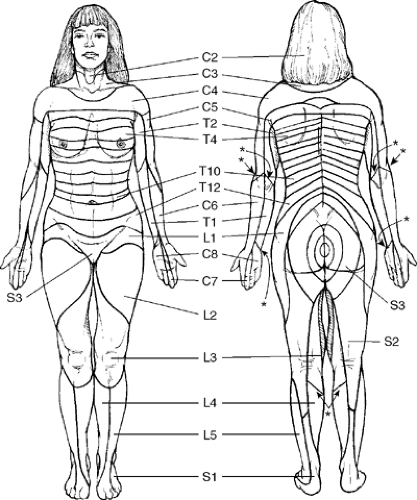
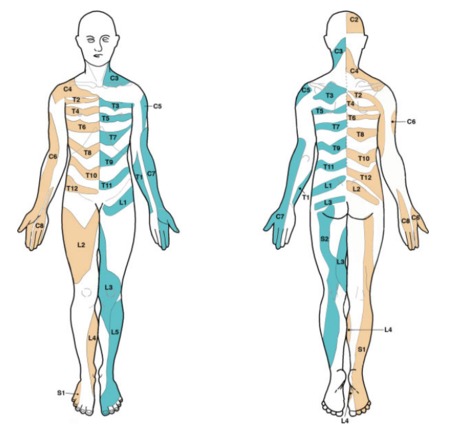
[[File:dermatome map lee.PNG|thumb|right|Dermatome map constructed by Lee and colleagues from research by other authors.<ref name="lee"/>|450x450px]] | [[File:dermatome map lee.PNG|thumb|right|Dermatome map constructed by Lee and colleagues from research by other authors.<ref name="lee"/>|450x450px]] | ||
The term dermatome generally refers to an area of skin innervated by a particular neural element, specifically nerve root, dorsal root ganglion or spinal segment. These are distinct from the cutaneous nerve distributions. | The term dermatome generally refers to an area of skin innervated by a particular neural element, specifically nerve root, dorsal root ganglion or spinal segment. These are distinct from the cutaneous nerve distributions. | ||
Revision as of 17:05, 17 April 2022
The term dermatome generally refers to an area of skin innervated by a particular neural element, specifically nerve root, dorsal root ganglion or spinal segment. These are distinct from the cutaneous nerve distributions.
Dermatome Maps
Methods of Investigation
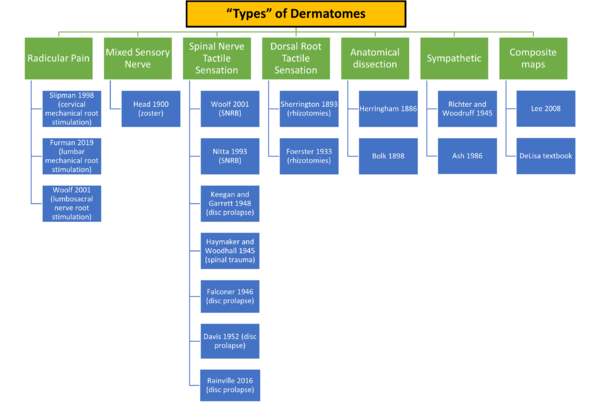
Before outlining the research into dermatome mapping, it is important to keep in mind that there are different "types" of dermatomes depending on what is being tested and how it is being tested.
There are a variety of ways of investigating dermatomes in a research setting.
- Gross dissection and mapping the nerves
- Making precisely placed spinal cord lesions – “remaining sensibility” and “constructive method”
- Single nerve root blocks
- Electrical nerve root stimulation witnessing vasodilation and pain
- Pain with needle irritation of nerve root
- Hypo-algesia in spinal nerve root compression
- Hyperalgesia with herpes zoster
The term "remaining sensibility" refers to cutting several nerve roots above and below the target nerve root for testing. The term "constructive method" refers to cutting multiple nerve roots below the target nerve root in one individual to determine the inferior border, and then in another patient cutting multiple nerve roots above to determine the superior border. See Downs et al on the history of dermatome maps.[2]
Primary Research
Bolk 1898 and Herringham 1886
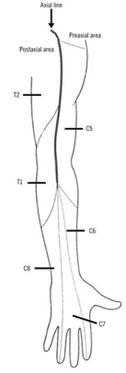
Much of the initial research was done through anatomical dissection by Bolk and Sir Wilmot Herringham. They followed fibres of single roots through their plexuses, peripheral nerves, and to skin. Herringham assessed the upper limb, and Bolk extended the work to the lower limb.
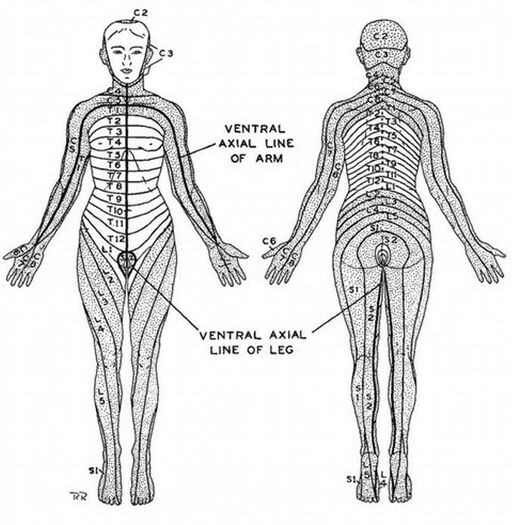
The authors described an axial line, with the areas next to the line being denoted as pre-axial and post-axial. They noted that the dermatomes in the preaxial area (C5-6) are higher than those in the post axial area (C8-T1). In the preaxial area the forearm (C6) is supplied by a lower nerve than the arm (C5), in the post axial area the forearm (C8) is supplied by a higher nerve than the arm (T1-2)
The main limitation of this method is that it is impossible to follow the terminal and finest ramifications of the sensory nerves. Herringham published the first account detailing the upper limb.
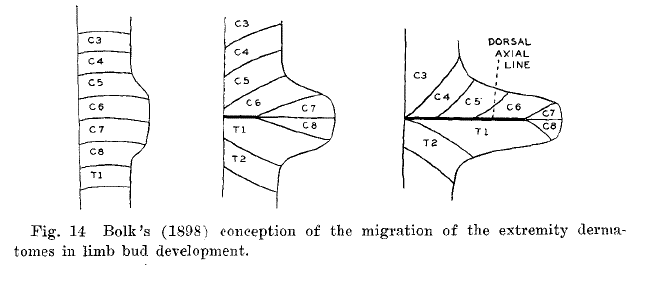
Bolk's theory was that the dermatomes shift in an insular form to the peripheries as the extremities grow, termed the "loop theory."
Charles Sherrington 1893, 1898
Sherrington published work in 1893 and 1898 done on rhesus monkeys where he severing several dorsal roots above and below the nerve being studied. This resulted in a dermatome with normal sensation bound on either side by anaesthetic areas (remaining sensibility).
He found that cutting a single nerve root didn't cause any sensory loss, that there was extensive dermatomal overlap, and that in the proximal portions there is a gap with missing contiguous dermatomes with no overlap.[3]
Henry Head 1892, 1900
Head, after initially publishing work on viscero-somatic referred cutaneous tenderness in 1892, subsequently published seminal work in 1900 with Campbell on dermatome mapping derived from patients with shingles. At the time doctors knew that shingles was related to chicken-pox and that it involved a lesion of a single dorsal root ganglion, but it was before the discovery of viruses in general.
He documented 450 cases of shingles looking for hyperalgesia and vesicular eruption. He includes 8 segments in 16 post-mortems (T1-L1). He used conjecture for much of the mapping work. Some of the observations he made included that there was minor overlap between adjacent territories, with overlap being more extensive in the limbs; there were inter-individual differences including with age and body shape; and post- and pre-fixed plexuses could change the pattern.[4]
This work uniquely reported on the thoracic dermatomes. However there was an important lack of histological confirmation with limb involvement. There were assumptions made about which segment was involved. We now know that sometimes several adjacent levels can be affected. Also not all cutaneous fibres will necessarily be infected.
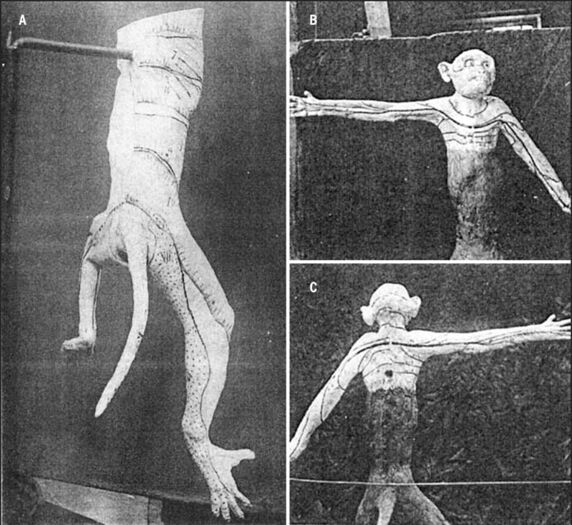
Otfrid Foerster 1933, 1936
In the early 20th century, rhizotomy of the dorsal roots was used to treat intractable pain from various conditions such as tabes dorsalis, cerebral palsy, and CNS trauma. Foerster therefore extended Sherrington's work to humans.[5]
He severed contiguous dorsal roots in patients with various painful conditions and documented the results of 30 years of experimental data. He used both the constructive and remaining sensibility methods. He assessed the dermatomes using tactile, thermal, pain stimuli, as well as electrical stimulation of dorsal roots producing cutaneous vasodilation.
He found that cutting a single nerve root didn't result in any sensory loss, except for C2. The dermatomes mapped from tactile stimuli were larger than that of pain and temperature stimuli. He also found variations between individuals and overlap.
His work is very important as ethically it is likely to never be reproduced. Unfortunately the documentation standards of the time were poor and his work was no different in this regard. There was unclear documentation regarding the interval between sectioning and testing, and the general methodology was poorly documented. He apparently assessed for complete anaesthesia not hypoaesthesia. With respect to clinical applications, we find that the extent of his dermatomes is greater than that usually involved by pathology.
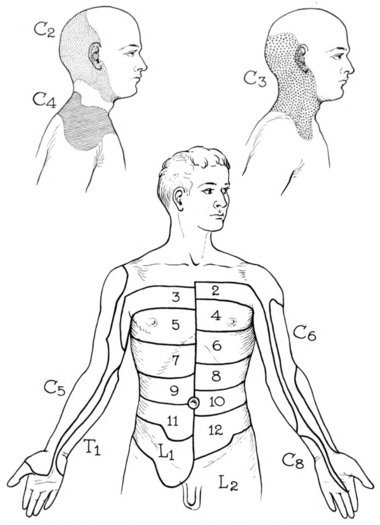
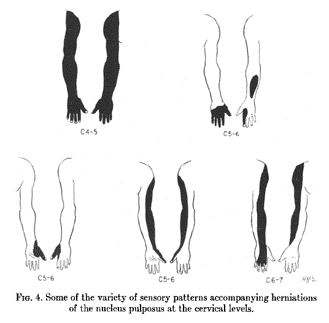
Foerster didn't present a single dermatome map, rather he presented multiple pictures of different patients with the sensory outlines drawn on their bodies. He did present a unified thoracic dermatome map, and some publications incorrectly state that this was his complete map. Texts often furthermore incorrectly state that he didn't assess the cervical dermatomes, which he definitely did. A few years after Foerster, Fender reinterpreted the work into dermatome illustrations as below. Lee in 2008 did the same but in one unified picture.
Haymaker and Woodhall 1945
Haymaker and Woodhall presumably based their map off treatment of wartime casualties during the American wartime experience. It was in turn based off data from the Medical Research Council pamphlet from 1942-3 which was in turn presumably based off the British wartime experience. They also reference Foerster but do not have Foerster's overlapping regions.
The work was published in a book which I have been unable to obtain.[6] It was apparently a handbook, not a research publication, but it was nevertheless extensively reproduced.
Keegan and Garrett 1948
Keegan and Garrett's map is arguably the most influential dermatome map to date. It was based off experimental data in patients with single nerve root compression due to disc prolapse. The series had 165 upper limb and 1264 lower limb patients.[7]
They assessed for hypoaesthesia to light pin-scratch. They plotted the "true primitive dermatome" not the completed distribution. They acknowledged overlap but stated that there was less overlap when assessing only the true primitive areas.
They found that proximal extensions had less dense sensation reduction. They thought previous researchers hadn't found the proximal extensions due to not looking for more subtle findings. The proximal findings meant that they thought Bolk's loop theory was wrong.
They verified their findings by a mixture of operative findings (707 patients), traction on exposed nerve roots, occasionally sectioning a nerve root, and injecting novocaine into single nerve roots in 10 medical students.
They asserted that Foerster and Sherrington were wrong about division of a single nerve root not causing sensory loss. In my view, clearly nerve root compression by disc prolapse and operative severing produce different changes.
There are several limitations to Keegan and Garret's data. The cervical dermatomes were only verified with myelography not surgery. Some dermatomes were not assessed yet appear in the chart for example the truncal dermatomes, L1, and L2. It is difficult to know how they determined whether only one nerve root was affected. It is hard to believe that they found highly universally reproducible dermatomes with little variability and little overlap between individuals. They didn’t present numerical data to support this. It is often said that ribbon patterns don’t fit with other data such as Davis in 1952 but that’s not completely fair as the lower limb is quite similar to Nitta et al's research. Other data show that there is better accuracy distally than proximally. For their nerve blocks, they used 2mL of 2% lidocaine which may have anesthetised adjacent nerve roots.
Davis 1952
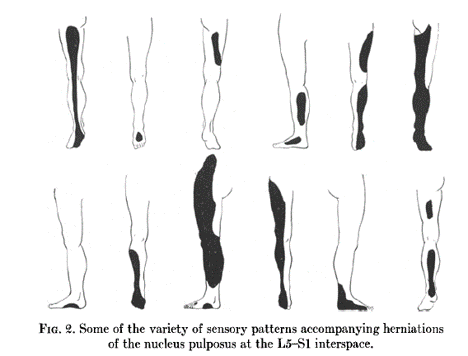
The work of Davis is often quoted as being in conflict to Keegan and Garrett. They presented data from 500 patients with disc protrusions, - 18 cervical, 201 L4-5, 234 L5-S1, and the remainder in other locations. They considered patients with transitional vertebrae separately.[8]
They found that 327 out of 500 had sensory changes. However they found no consistent pattern. The patterns were highly variable. For example L4-5 had 38 patterns, L5-S1 had 29 patterns. Over half had foot and leg involvement only. They also noted inter-observer variability.
The explanation of their methodology is limited by today's standards. It isn't certain how they determined what a "pattern" was for example. They didn't present data related to certain anatomical landmarks, for example how many patients had changes of the lateral foot in S1 compression.
Primary Research in More Limited Dermatomes
Falconer 1946
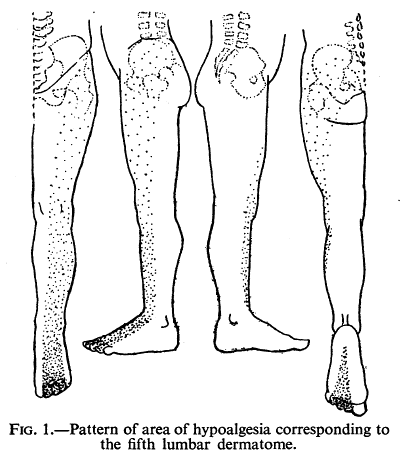
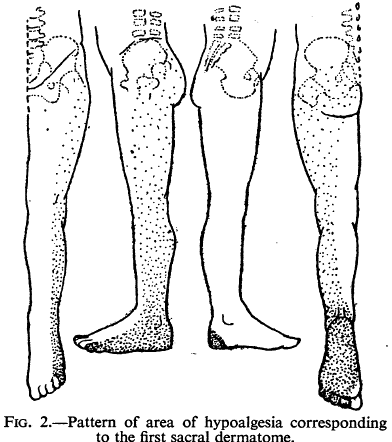
Falconer published shortly before Keegan's seminal work, but some early data of theirs had already been published. The assess for hypoalgesia to series of pin pricks found in 33 of 50 consecutive patients with L5 or S1 nerve root lesions. They also did nerve interlaminar root blocks in a few patients.[9]
They commonly commonly found changes in thigh and buttock, supporting the ribbon view. But they noted extensive overlap unlike Keegan and Garrett.
Nitta 1993 and Wolff 2001: Nerve root blocks in patients with lumbar radicular pain
Nitta reported on 71 patients with lumbosacral radicular symptoms having 86 spinal nerve blocks with 1.5mL 2% lidocaine under fluoroscopy. They looked for for regions of sensory impairment using the writing brush (tactile) method, which was traced, photographed, and graphed. They excluded patients with transitional vertebrae.[10]
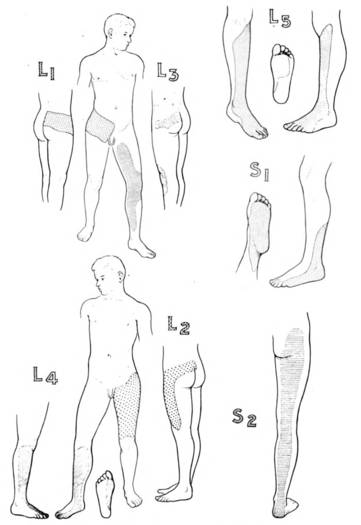
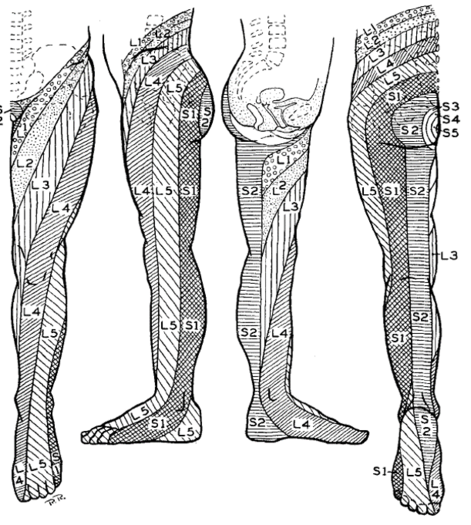
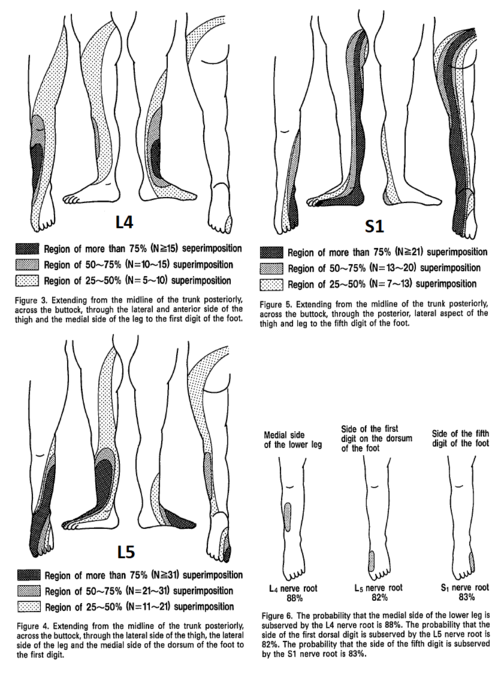
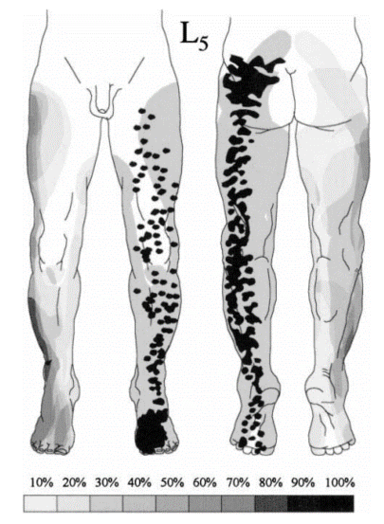
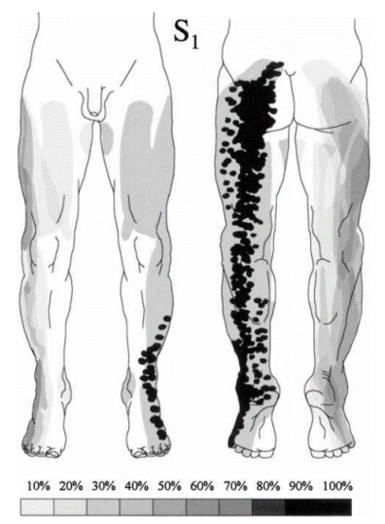
Nitta was aware of the ribbon vs chunk debate. He found ribbon's in in 42% of L4, 44% of L5, and 92% of S1. hey also reported on the prevalence of distinctive regions. The medial side of the lower leg was subserved by L4 in 88%, the side of the first dorsal digit was subserved by L5 in 82%, and the side of the fifth digit was subserved by S1 in 83%.
Nitta's dermatome map of L4-S1 is remarkably similar to Keegan and Garrett's. Some of the literature that synthesises the history of dermatomes incorrectly state there is no other experimental data validating Keegan and Garrett's work (e.g. the DeLisa textbook).
The main limitation of their work is the that they didn’t describe any control of anaesthetic spread, and that it was limited to three dermatomes.
Wolff et al did 42 selective lumbosacral nerve root blocks in 29 patients with radicular pain without neurological deficit. They recorded paraesthesias by electrical stimulation, and Hypoaesthesia (pinprick) after 1mL 1.5% lidocaine injection.[11]
They compared to Keegan and Garret and their own adapted map with overlap of 50% of adjacent segments. They found that in most cases hypo-aesthesia after single nerve root block was found in at least 2 dermatomes (range 1-6). They found many ribbon patterns but there was extensive overlap.
The areas of hypoesthesia were only in agreement with Nitta for S1. L4 involved the anterolateral knee; L5 was the lower leg and lateral ankle; and S1 was the dorsolateral thigh, lateral ankle, lateral foot. They found more variability with hypoaesthesia than paraesthesia
They used a blinded investigator, assessed for injectate spread, and only assessed in patients with a normal number of vertebrae.
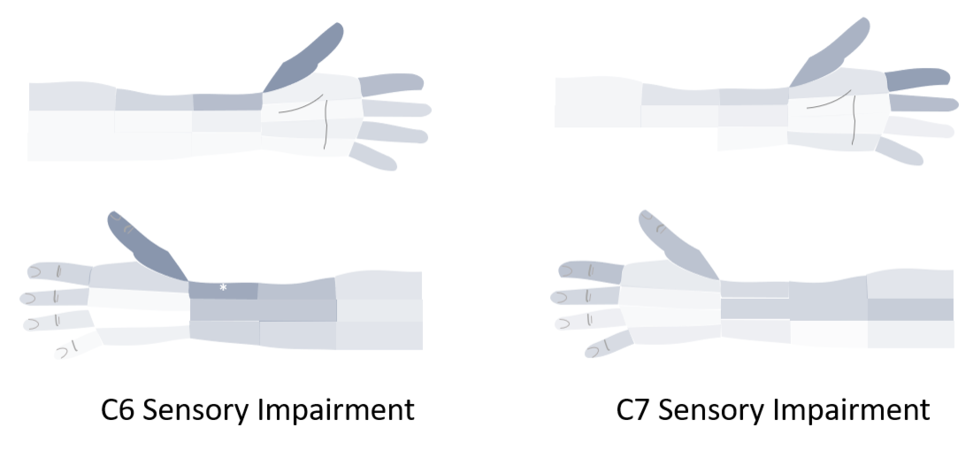
Rainville 2016: Impaired sensation in cervical radiculopathy
Rainville et al assessed for impaired pinprick sensation in 30 patients with C6, and 40 patients with C7 MRI confirmed cervical radiculopathy. The sensory exam was done before the MRI and before the motor reflex exam by 11 different clinicians and drawn onto a preset grid.[12]
The findings were 80% had impaired sensation in at least 1 grid, most often distally. There was almost complete overlap of C6 and C7 dermatomes. The dorsal aspect of distal radial forearm impairment twice as common in C6 (43% vs 18%), indicated by the asterix below. However essentially it isn't possible to differentiate C6 and C7 radiculopathy on the sensory exam. This is consistent with anatomical data showing that the majority of anastomoses occur between C6 and C7.[13]
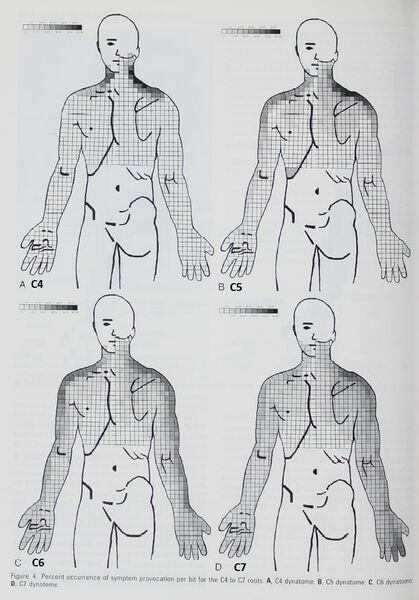
Slipman 1998 and Furman 2019: Pain with mechanical nerve root stimulation
Slipman did fluoroscopically guided cervical nerve root purposeful mechanical stimulation of 134 roots in 87 patients and recorded the pattern of pain. They called the resulting pain maps "dynatomes."[14]
They found that in a significant portion of individuals, pain patterns did not follow dermatomal patterns. In fact, no pattern was confined to classic dermatomes in over 50% of patients. Many of the patterns were quite surprising. For example symptoms in all five fingers occurred in 12% of C6, 10% of C7, and 5% of C8. It is difficult to summarise, but the most clinically useful C6 and C7 findings are below, of which they had the most patients. It is interesting to note how similar the distal findings are between these two levels.
- They found that C5 was distal to elbow in 14%, C6 affected the ulnar hand in 67%, C7 uniquely resulted in anterior head symptoms and referred to chest, and C8 referred to the thumb in only 14%. They found that no pattern was confined to classic dermatomes in over 50% of patients.
- They found that C5 was distal to elbow in 14%, C6 affected the ulnar hand in 67%, C7 uniquely resulted in anterior head symptoms and referred to chest, and C8 referred to the thumb in only 14%. They found that no pattern was confined to classic dermatomes in over 50% of patients.
Furman's study was essentially looking at the lumbar "dynatomes." They had 71 patients having 125 supraneural TFIs. Unlike slipman, they didn't purposefully stimulate the nerve root, but recorded the findings if there was inadvertent induced radicular pain. 87 of their cases had induced pain. They excluded patients with transitional vertebrae.[15]
They found that referred pain to the buttock, posterior thigh or posterior calf may come from L3, L4, L5, or S1 nerve roots segmental irritation. Referral to to anterior, medial, lateral thigh, and leg often followed predicted map. Unfortunately they didn't attempt to chart the findings onto a grid like Slipman and so there is no picture.
| Nerve Root | L3 | L4 | L5 | S1 |
|---|---|---|---|---|
| Buttock | 45% | 43% | 62% | 64% |
| Groin | 0% | 3% | 0% | 0% |
| Anterior Thigh | 27% | 29% | 12% | 0% |
| Posterior Thigh | 36% | 25% | 59% | 36% |
| Medial Thigh | 18% | 11% | 3% | 0% |
| Lateral Thigh | 0% | 14% | 9% | 0% |
| Knee | 9% | 7% | 6% | 9% |
| Anterior Leg | 0% | 14% | 3% | 0% |
| Posterior Leg | 18% | 18% | 50% | 45% |
| Medial Leg | 0% | 7% | 6% | 0% |
| Lateral Leg | 9% | 14% | 24% | 0% |
| Foot | 0% | 3% | 0% | 0% |
Composite Map
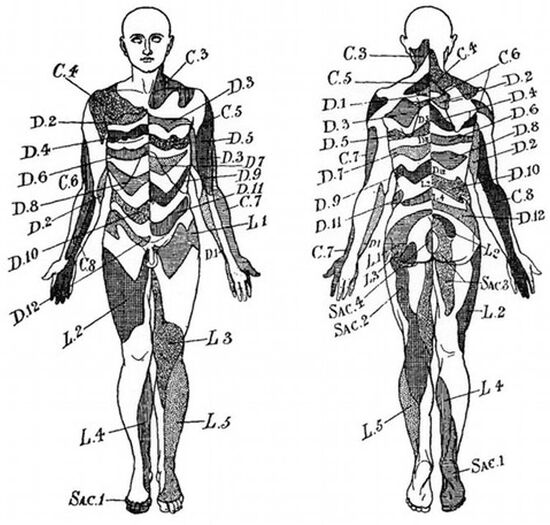
The most up to date dermatome map is probably that published by Lee et al in 2008. They created a composite image taken from published data that they thought was the most reliable. [1]
They redrew the Head and Campbell, and Foerster maps from the original photographs onto a figure outline. The figures were overlaid, and the areas that did not overlap were deleted. This new template was then modified in the light of other good category evidence data for C6, C7, and C8 (Inouye and Buchthal, 1977) and L4, L5, and S1 (Cole et al., 1968; Nitta et al., 1993). They considered Keegan and Garrett's map to be the most flawed of all dermatomal maps and it was not included.
They found that there is greater lumbosacral variability is greater than cervical. Adjacent dermatomes overlap to a variable degree. Dermatomes in the hand overlap to a large degree. Variability may be explained by the following reasons: almost all areas of the skin are supplied by two or more spinal nerve roots; Intrathecal intersegmental anastamoses are common; the distribution of a dorsal root is slightly different to that of a dorsal root ganglion; and these areas are influenced by spinal cord processing. Dermatomes vary based on the method of assessment, the type of sensation tested, and the precise neural element being tested
Lee et al's map is the most consistent for tactile dermatomal regions for each spinal dorsal nerve root. The midline has minimal overlap, but otherwise there is extensive and variable overlap. Blank areas on the map represent areas where there is very large degree of variability and overlap. The S3, S4, and S5 cutaneous supply is not shown.
Embryology
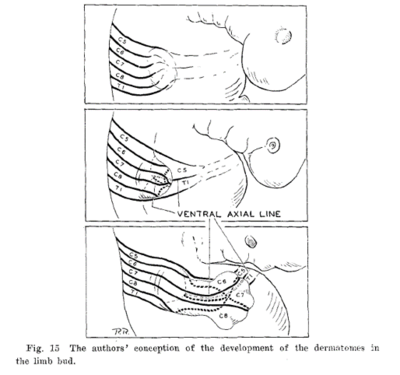
The Haymaker and Woodhall type of dermatome chart presents regions of spinal segments having an insular or "chunk-like" form. The Keegan and Garrett type shows regions with continuous "ribbon-like" zones extending proximally to distally. These difference relate to differences in opinion regarding the embryology of dermatomes in the extremities. [16]
Bolk's loop theory was that the dermatomes shift in an insular form to the periphery as the extremities grow. In the loop theory, there is simple outward bulging of already delimited trunk dermatomes. The dermatomes are carried in the form of loops over the expanding limb buds. There is a separation of dermatomes from the midline.
Keegan and Garrett's theory was that there is a free course of outgrowing fibres through an already formed nerveless limb bud. They referenced work done in limb bud development in the frog to support this theory. This developmental theory would explain the presence of dermatome bands.
The current developmental thinking from what I can see is that Keegan and Garrett were basically right. Nerves are not present at all in early limb buds. At a later data plexuses form, and then the nerves grow more-or-less directly to their appropriate targets, with motor nerves being earlier than sensory nerves. There is no major rearrangement.[17]
Bolk (1898) was actually proven wrong by Lewis in 1902, and this substantiated by later research especially on chicken fetuses. Texts within a few years afterwards tended to follow Lewis's theory, but then all of a sudden Lewis was forgotten with no evidence to substantiate Bolk’s view.
Some embryology texts are still incorrect, for example Moore's clinically oriented embryology published in 2016 is unreferenced but appears to continue to promote Bolk's disproven theory.
Synthesis and Concept Validity
Definition
There are two conflicting definitions of dermatome.
- Embryological definition: Somite (mesodermal structure) that became part of the dermis, where the somites are spatially distinct
- Mature definition: The entire area of skin (epidermis plus dermis) which receives its sensory innervation from the dorsal root of a single spinal nerve, where the dermatomes overlap considerably.
Semantically the embryological definition is more consistent with other analogous terms such as myotome and sclerotome. The mature definition is more accurate but actually refers to segmental changes.
The usual "embryological" and "mature" definitions are not appropriate for maps nor are they useful clinically. What is in fact clinically useful is knowing the location and distribution of changes that are associated with pathological segmental processes in the spinal nerves, their dorsal roots, and the corresponding levels of the spinal cord.
Clinical Correlation
There are different types of dermatomes e.g. “tactile dermatomes”, “somatic pain dermatomes”, “radicular pain dermatomes”, “sympathetic dermatomes”, “vasomotor dermatomes." For example pain or thermal dermatomes are usually smaller than corresponding tactile dermatomes. Thermal dermatomes are often smaller than pain dermatomes. The boundaries differ depending on the stimulus and criteria used.
Clinical changes are generally either ablative or irritative. Ablative processes such as nerve root compression result in negative findings e.g. reduced sensation to light touch and pin prick. Irritative processes such as nerve root irritation result in spontaneous hypersensitivity to different stimuli. Infections such as herpes zoster, and developmental disorders such as vascular/pigmented nevi are another group that cause structural changes.[18]
In essence, there are multiple conflicting candidates for the “true” dermatome. It is important to keep in mind what you are trying to correlate it to i.e. cutaneous sensory loss, superficial pain, radicular pain, or deep somatic pain. With deep somatic pain in particular, pain patterns are likely to more closely follow myotomal patterns than dermatomal patterns.
Expansion and Contraction
Dermatome maps are a simplification of what happens in reality. Dermatomes in reality are irregularly shaped and highly overlapping areas of skin that belong to 3-5 different dermatomes. They are dynamic, can expand and shrink, have inter- and intra-individual variations, and can vary depending on the precise structure being tested and the method of testing as mentioned above.
The dermatome concept is based on the assumption that there is a direct and static correlation between nerve supply and skin sensation. Nerve signals are in fact highly dynamic. Nerve fibres are less dense towards the periphery of a dermatome, so dermatome maps may actually reflect maximum innervation. Cutaneous innervation of the dorsal root ganglia is different to that of the dorsal root as discovered through sectioning monkey nerves proximal and distal to the dorsal root ganglion. The Lissaeur tract can facilitate and suppress sensory impulses, corresponding to dermatomal expansion or reduction. In my own clinical experience, patients have different areas of hypoaesthesia on different days of testing.
The research basis for this is the work done by Denny-Brown et al published between 1968-1973.[19][20][21] This work had a profound influence on the understanding of dermatomes. It is complex, which may be why it has been practically ignored in more literature and textbooks. The work has been supported by further research.
They researched on monkeys. In their dorsal root ganlglia research, they assessed the remaining sensibility after sectioning 3 roots above and 3 roots below the target root. The also sectioned roots just distal or proximal to the DRG. In both of these experiments, there was only one DRG serving sensory function over region spanning 7 roots They found
- Dorsal root ganglia and dorsal roots had different dermatome patterns.
- Dermatomes could expand in size with physiological but not anatomical change
- Discrete lesions of spinal cord, even caudal to the root, could dramatically alter the size of the dermatome
- Neighbouring DRG facilitate sensory transmission of given DRG. With distal sectioning the dermatomes were smaller, with subcutaneous strychnine injection there was expansion of isolated dermatome (strychnine interferes with spinal inhibition)
In their Lissaeur tract research they found the following
- Lissauer tract mediates spinal effects on dorsal root transmission.
- Medial portion facilitates sensory function, lesions here cause dermatomes to shrink, and strychnine after sectioning causes re-expansion.
- Lateral portion inhibits sensory function (lesions cause dermatomes to expand)
Anatomical Variations
There are many very common anatomical variations that will also affect dermatomal mapping.
- Intrathecal intersegmental anastamoses between the dorsal spinal rootlets [22][23], with the greatest variation in upper limb dermatomes
- Postfixed brachial plexus (large contribution from T2 with or without a small C5 contribution)[24]
- Prefixed brachial plexus (large contribution from C4 with or without a small T1 contribution)[24]
- The furcal nerve. Furcal means forked. This is an independent nerve with its own ventral and dorsal branches (rootlets) and it forms a link between the lumbar and sacral plexuses. It is most commonly found at L4, with L3 being the next most common site. This can cause an atypical presentation of sciatica.
- Transitional lumbosacral anatomy may result in the L4 nerve root serving the usual function of L5 in L5 sacralisation, and the S1 nerve root serving the usual function of L5 in S1 lumbarisation.[16]
- Intra-individual (side to side) differences
Sensory Fibre Transits
Not all sensory fibres in a given spinal nerve continue into a corresponding dorsal root, and not all fibres in a given dorsal root come from the corresponding spinal nerve. There are numerous connections between dorsal rami of spinal nerves forming a continuous plexus where fibres can run upwards or downwards before entering a different dorsal root. There are also connections between dorsal root ganglia, dorsal roots, and between roots and ganglia.
In monkeys sensory axons in any given dorsal root can come from at least five different spinal nerves, likewise any point on the body is innervated by axons from at least five dorsal roots. Cutaneous innervation of the dorsal root ganglia is different to the dorsal root.
Overlap
Dermatome maps can be divided into non-overlapping and overlapping types. The two main non-overlapping types are the Haymaker and Woodhall 1945 map, and the Keegan and Garrett 1948 map. The main overlapping type is that of Foerster 1933. Head's map of 1900 didn't show overlap for simplicity but said there was overlap. The evidence overall indicate that overlap does occur.
There are some conclusions we can make with respect to "autonomous regions." The most important clinically useful dermatomes have been studied to answer this question. The evidence show that this is an invalid concept for C6 and C7 differentiation.[12] However for the L4-S1 dermatomes in the lower limbs this may be a slightly useful, keeping in mind that the autonomous regions are not universal.[10] There are no clear data to either support or refute the concept of autonomous regions for the thoracic dermatomes.
Mixed Pain Processes
For “pain dermatomes” it is uncertain how to distinguish between mixed somatic and radicular pain. In sciatica, there may be an associated somatic process from dural sleeve irritation or from an associated annular tear
Is Head’s 1900 dermatome map a mixed “dorsal root ganglion dermatome map” and “dorsal root/spinal nerve dermatome map”
Unlike the trunk, in the limbs skin innervation follows the cutaneous nerves, while the muscles show a different pattern. This is termed deep somatic pain, and the patterns of pain are different to that seen with cutaneous pain.
| Upper Limbs | Lower Limbs | |
|---|---|---|
| Anterior Compartment | C5 | L4 |
| Lateral Compartment | C6 | L5 |
| Posterior Compartment | C7 | S1 |
| Medial Compartment | C8 | L3 |
Ribbons vs Chunks
It is clear that ribbon-like patterns of hypoaesthesia can occur with disc prolapse (Keegan and Garrett, Nitta, Falconer, Wolff). However this does not always happen, and sensory changes may be greatest distally
Several theories have been put forward for why some individuals but not others have ribbon like patterns in lumbosacral radicular pain.
- Some individuals have cutaneous branches of dorsal rami to the posterior trunk. L4 (in 4/10 embryo specimens) can be direct. L5 and S1 often join the posterior sacral plexus.[10]
- The lateral femoral cutaneous nerve (L2-3) overlaps the posterior lateral thigh that is sometimes covered by L4 and L5. In individuals with dense overlap, there would be no reduced sensation with L4 or L5 block causing an insular dermatome.[10]
- The lumbosacral region and buttock share a similar segmental nerve supply (L4-S1). The low back is innervated by the dorsal rami of L4-S1 and the deep buttock by the ventral rami. Stimulation of the sinuvertebral nerves that innervate the dural sleeve may be a source of somatic referred pain.[26]
- With nerve root stimulation, muscular contraction is produced. This muscular action serves to move the bone but also stretch the overlying deep fascia due to the presence of myofascial expansions. Pain in the buttocks, posterior thigh, or posterior calf, could be due to excessive tension of the deep fascia along a specific line of force, activating all the free nerve endings along that line.[25]
Lumbar Descending vs Exiting Roots
It isn't clear whether the authors looking at lumbar disc prolapse in the 40s and 50s (Davis, Falconer, Keegan and Garrett) understood about descending vs exiting root compression states. I have reviewed their work and it appears that they only considered descending root compression in the lateral recess, not foraminal compression from a far lateral disc which would compress the exiting nerve root. They used myelography seemingly to determine the level of disc prolapse, rather than looking for lack of nerve root filling. Therefore as Slipman et al point out, it is possible that they will not have accounted for cases of foraminal stenosis, lateral, extra-foraminal, and foraminal hernations, or sequestered or migrated disc fragments.
Clinically Testing Dermatomes
The area of testing will be determined by the history. It is not necessary normally to test all dermatomes. Ask the patient where they have altered sensation and its borders. Dermatomes can be sampled by moving distal to proximal. Move along the long axis of the medial and lateral aspects of the upper and lower limbs, and caudal to cranial along both sides of the trunk.
Test for pin prick sensation in the dermatomes. Temperature sensation can be tested, but this is usually omitted if pinprick sensation is normal. Cold sensation can be tested by using the metal of a tuning fork. Light touch adds little additional information, and is usually tested with a wisp of cotton wool or a light finger touch to the skin. The area of deficit to light touch is often larger than that of pin prick.
If an area of sensory deficit is detected to pinprick, then examine from the centre of maximum deficit and move outwards to define the borders. If there is an area of increased sensation (e.g. hyperalgesia), then the mapping should be done in the reverse direction outwards to inwards.
It is not possible to distinguish between adjacent dermatomes (e.g. between C6 and C7), but it may be possible to distinguish between non-adjacent dermatomes. (e.g. between C5 and C7)
ASIA Landmarks
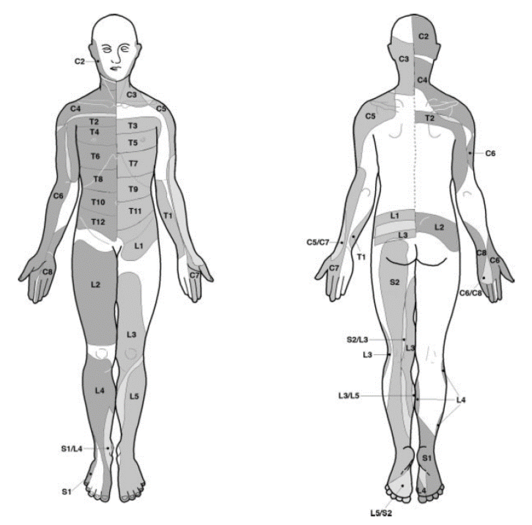
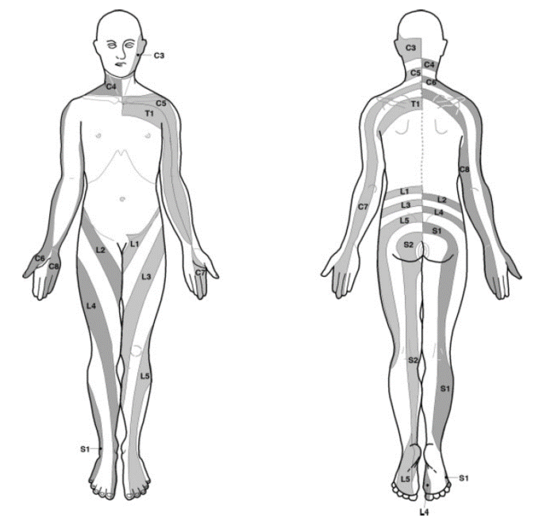
Dermatomal landmarks are commonly used and are based on ASIA's publication, called "key sensory points," otherwise known as "autonomous regions." The landmarks are highly questionable as it is not actually apparent how ASIA determined them, and the primary research does not support the concept of autonomous regions. The Overall ASIA map bears very close resemblance to the Haymaker and Woodhall map.
| Nerve root | ASIA | Comment |
|---|---|---|
| C2 | At least one cm lateral to the occipital protuberance at the base of the skull. Alternately, a point at least 3 cm behind the ear. | |
| C3 | In the supraclavicular fossa, at the midclavicular line. | |
| C4 | Over the acromioclavicular joint. | |
| C5 | On the lateral (radial) side of the antecubital fossa, just proximally to the elbow. | This is C6-C7 based on Lee. Autonomous region should be regimental badge area |
| C6 | On the dorsal surface of the proximal phalanx of the thumb. | Considerable overlap with C7 |
| C7 | On the dorsal surface of the proximal phalanx of the middle finger. | Considerable overlap with C6 and C8 |
| C8 | On the dorsal surface of the proximal phalanx of the little finger. | Considerable overlap with T1 |
| T1 | On the medial (ulnar) side of the antecubital fossa, just proximally to the medial epicondyle of the humerus. | |
| T2 | At the apex of the axilla. | |
| T3 | Intersection of the midclavicular line and the third intercostal space | |
| T4 | Intersection of the midclavicular line and the fourth intercostal space, located at the level of the nipples. | |
| T5 | Intersection of the midclavicular line and the fifth intercostal space, horizontally located midway between the level of the nipples and the level of the xiphoid process. | |
| T6 | Intersection of the midclavicular line and the horizontal level of the xiphoid process. | |
| T7 | Intersection of the midclavicular line and the horizontal level at one quarter the distance between the level of the xiphoid process and the level of the umbilicus. | |
| T8 | Intersection of the midclavicular line and the horizontal level at one half the distance between the level of the xiphoid process and the level of the umbilicus. | |
| T9 | Intersection of the midclavicular line and the horizontal level at three quarters of the distance between the level of the xiphoid process and the level of the umbilicus. | |
| T10 | Intersection of the midclavicular line, at the horizontal level of the umbilicus. | |
| T11 | Intersection of the midclavicular line, at the horizontal level midway between the level of the umbilicus and the inguinal ligament. | |
| T12 | Intersection of the midclavicular line and the midpoint of the inguinal ligament. | |
| L1 | Midway between the key sensory points for T12 and L2. | |
| L2 | On the anterior medial thigh, at the midpoint of a line connecting the midpoint of the inguinal ligament and the medial epicondyle of the femur. | |
| L3 | At the medial epicondyle of the femur. | |
| L4 | Over the medial malleolus. | Medial side of the lower leg in 88%.[16] |
| L5 | On the dorsum of the foot at the third metatarsophalangeal joint. (Note hallux can be supplied by L4) | First dorsal digit in 82%[16] |
| S1 | On the lateral aspect of the calcaneus. | lateral side of 5th digit in 83%.[16] Overlaps with S2 |
| S2 | At the midpoint of the popliteal fossa. | |
| S3 | Over the tuberosity of the ischium or infragluteal fold | |
| S4 and S5 | In the perianal area, less than one cm lateral to the mucocutaneous zone |
Literature
- Media:The_pathology_of_herpes_zoster_and_its_bearing_on_sensory_localisation_republication_of_selected_parts_-_head_1900.pdf
- Media:Foersters_scheme_of_dermatomes_-_fender_1939.pdf
- Media:L5_and_S1_sensory_changes_due_to_lumbosacral_disc_protrusions_-_falconer_1946.pdf
- Media:The_segmental_distribution_of_the_cutaneous_nerves_in_the_limbs_of_man_-_Keegan_and_Garret_1948.pdf
- Media:Sensory_changes_with_herniated_nucleus_pulposis_-_Davis_1952.pdf
- Media:An_analysis_of_the_lumbosacral_dermatomes_in_man_-_Cole_1968.pdf
- Denny-Brown et al: 1968 article, 1970 article, 1973 article
- Media:C2_and_C3_pain_dermatomes_in_man_-_Poletti_1991.pdf
- Media:Study_on_dermatomes_by_means_of_selective_lumbar_spinal_nerve_block_-_Nitta_1993.pdf
- Media:Symptom_provocation_of_fluoroscopically_guided_cervical_nerve_root_stimulation_-_slipman1998.pdf
- Media:Diagnostic_Lumbosacral_Segmental_Nerve_Blocks_-_Wolff_2001.pdf
- Media:Exploration_of_sensory_impairments_associated_with_C6_and_C7_radiculopathies_-_Rainville_2016.pdf
- Media:Induced_lumbosacral_radicular_symptom_referral_patterns_-_furman_2018.pdf
Summary
- Texts on the subject of dermatomes are littered with inaccuracies, falsified data, poor methodology, and propagation of incorrect data through consecutive editions of textbooks.
- Dermatomes are not static but rather neurophysiological entities.
- There are different "types" of dermatomes depending what is tested and method of testing
- In individual patients there may be “autonomous regions,” but they cannot be used to differentiate adjacent dermatomes due to extensive overlap. However they may serve as a very rough guide for non-consecutive dermatomes. Autonomous regions are also less common in the upper limbs than the lower limbs.
- There are inter-individual differences due to profound neuroanatomical variability as well as changes with age and body shape.
- There are intra-individual differences
- Ribbon-like patterns of hypo-aesthesia can but don't always occur, but proximal extensions are less useful clinically as they are less marked in their changes.
- Dermatomes can expand and contract
References
- ↑ 1.0 1.1 Lee et al.. An evidence-based approach to human dermatomes. Clinical anatomy (New York, N.Y.) 2008. 21:363-73. PMID: 18470936. DOI.
- ↑ Downs & Laporte. Conflicting dermatome maps: educational and clinical implications. The Journal of orthopaedic and sports physical therapy 2011. 41:427-34. PMID: 21628826. DOI.
- ↑ Sherrington C. and Experiments in examination of the peripheral distribution of the fibres of the posterior roots of some spinal nerves. Philosophical Transaction of the Royal Society of London, Series B. 1893; 184: 641– 763.
- ↑ Head H, , Campbell AW. and The pathology of herpes zoster and its bearing on sensory localization. Brain 1900; 23: 353– 523.
- ↑ O. FOERSTER, THE DERMATOMES IN MAN , Brain, Volume 56, Issue 1, March 1933, Pages 1–39, https://doi.org/10.1093/brain/56.1.1
- ↑ Peripheral Nerve Injuries: Principles of Diagnosis - 1945
- ↑ KEEGAN JJ, GARRETT FD. The segmental distribution of the cutaneous nerves in the limbs of man. Anat Rec. 1948 Dec;102(4):409-37. doi: 10.1002/ar.1091020403. PMID: 18102849.
- ↑ Davis L, Martin J, Goldstein SL. 1952. Sensory changes with herniated nucleus pulposus. J Neurosurg 9:133–138.
- ↑ FALCONER MA, GLASGOW GL, COLE DS. Sensory disturbances occurring in sciatica due to intervertebral disc protrusions; some observations on the fifth lumbar and first sacral dermatomes. J Neurol Neurosurg Psychiatry. 1947 May;10(2):72-84. doi: 10.1136/jnnp.10.2.72. PMID: 20265613; PMCID: PMC497097.
- ↑ 10.0 10.1 10.2 10.3 Nitta H, Tajima T, Sugiyama H, Moriyama A. Study on dermatomes by means of selective lumbar spinal nerve block. Spine (Phila Pa 1976). 1993;18:1782-1786.
- ↑ Wolff AP, Groen GJ, Crul BJ. Diagnostic lumbosacral segmental nerve blocks with local anesthetics: a prospective double-blind study on the variability and interpretation of segmental effects. Reg Anesth Pain Med. 2001 Mar-Apr;26(2):147-55. doi: 10.1053/rapm.2001.21436. PMID: 11251139.
- ↑ 12.0 12.1 Rainville J, Laxer E, Keel J, Pena E, Kim D, Milam RA, Carkner E. Exploration of sensory impairments associated with C6 and C7 radiculopathies. Spine J. 2016 Jan 1;16(1):49-54. doi: 10.1016/j.spinee.2015.07.462. Epub 2015 Aug 4. PMID: 26253986.
- ↑ SCHWARTZ HG. Anastomoses between cervical nerve roots. J Neurosurg. 1956 Mar;13(2):190-4. doi: 10.3171/jns.1956.13.2.0190. PMID: 13307296.
- ↑ Slipman CW, Plastaras CT, Palmitier RA, Huston CW, Sterenfeld EB. Symptom provocation of fluoroscopically guided cervical nerve root stimulation. Are dynatomal maps identical to dermatomal maps? Spine (Phila Pa 1976). 1998 Oct 15;23(20):2235-42. doi: 10.1097/00007632-199810150-00019. PMID: 9802168.
- ↑ Furman MB, Johnson SC. Induced lumbosacral radicular symptom referral patterns: a descriptive study. Spine J. 2019 Jan;19(1):163-170. doi: 10.1016/j.spinee.2018.05.029. Epub 2018 May 22. PMID: 29800710.
- ↑ 16.0 16.1 16.2 16.3 16.4 Nitta et al.. Study on dermatomes by means of selective lumbar spinal nerve block. Spine 1993. 18:1782-6. PMID: 8235861. DOI.
- ↑ Mclachlan. Development of the dermatome. 1990
- ↑ Terence R Anthoney. Neuroanatomy and the Neurologic Exam: A Thesaurus of Synonyms, Similar-Sounding Non-Synonyms, and Terms of Variable Meaning. 1993
- ↑ Denny-Brown & Kirk. Hyperesthesia from spinal and root lesions. Transactions of the American Neurological Association 1968. 93:116-20. PMID: 4974855.
- ↑ Kirk & Denny-Brown. Functional variation in dermatomes in the macaque monkey following dorsal root lesions. The Journal of comparative neurology 1970. 139:307-20. PMID: 4317449. DOI.
- ↑ Denny-Brown et al.. The tract of Lissauer in relation to sensory transmission in the dorsal horn of spinal cord in the macaque monkey. The Journal of comparative neurology 1973. 151:175-200. PMID: 4355326. DOI.
- ↑ Moriishi et al.. The intersegmental anastomoses between spinal nerve roots. The Anatomical record 1989. 224:110-6. PMID: 2729613. DOI.
- ↑ Du Plessis et al.. Differentiation and classification of thoracolumbar transitional vertebrae. Journal of anatomy 2018. 232:850-856. PMID: 29363131. DOI. Full Text.
- ↑ 24.0 24.1 Pellerin et al.. The prefixed and postfixed brachial plexus: a review with surgical implications. Surgical and radiologic anatomy : SRA 2010. 32:251-60. PMID: 20087592. DOI.
- ↑ 25.0 25.1 Stecco C, Pirri C, Fede C, Fan C, Giordani F, Stecco L, Foti C, De Caro R. Dermatome and fasciatome. Clin Anat. 2019 Oct;32(7):896-902. doi: 10.1002/ca.23408. Epub 2019 May 28. PMID: 31087420.
- ↑ Bogduk, Nikolai. Clinical and radiological anatomy of the lumbar spine. Edinburgh: Elsevier/Churchill Livingstone, 2012
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,