Lumbar Radicular Pain and Radiculopathy
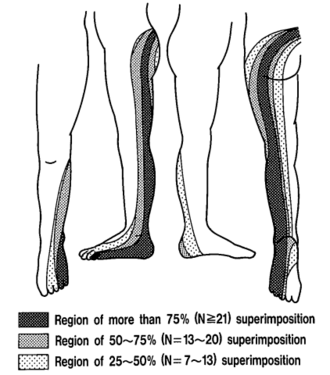
Radicular pain is generated by discharges from a dorsal root or dorsal root ganglion. Inflammation plays an important role as simple compression does not necessarily result in pain. Radicular pain may or may not be associated with radiculopathy. Disc herniation is the most common cause of radicular pain. The pain is often described as travelling along the length of the limb in a narrow band two to three inches wide.[1] L4, L5, and S1 pain patterns have significant overlapping pain areas.
Aetiology
In patients ~20-60 years old disc herniation is by far the most common cause of lumbar radicular pain. And even when viewed across all ages it is the most common cause. The nerve roots can be compressed anywhere along their length but this most commonly occurs in the ventral epidural space, lateral recess, and foramen.
In the elderly foraminal stenosis and spinal stenosis are important considerations. Nerve root compression is associated more with bony entrapment, and nerve root irritation is associated more with disc prolapse.
A large number of other conditions make up the remaining causes.
| Condition | Cause |
|---|---|
| Disc Disorders | Disc Herniation |
| Foraminal Stenosis |
|
| Epidural Disorders |
|
| Meningeal Disorders |
|
| Neurological Disorders |
|
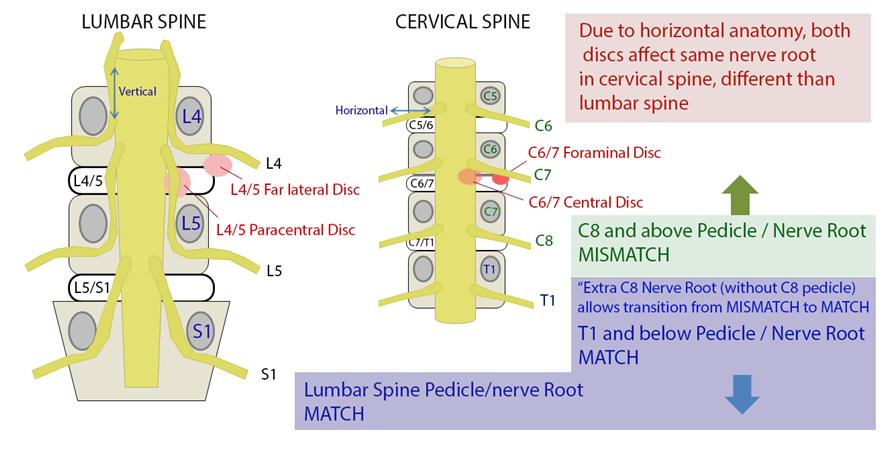
Lateral Recess Stenosis
In lateral recess stenosis it is the descending nerve root that is irritated or compressed. For example in L4/5 disc prolapse affecting the lateral recess, it is the descending L5 nerve root that is at risk not the exiting L4 nerve root.
Foraminal Stenosis
Less commonly the exiting nerve root is irritated or compressed in the foramen.
Pathophysiology
- See also: Radicular Pain and Radiculopathy
Radiculopathy occurs when conduction is blocked in the axons of a spinal nerve or its roots. When the sensory axons are affected the patient develops numbness, while when the motor axons are affected then weakness develops. Radiculopathy can be a result of compression or ischaemia. Radiculopathy does not cause pain in either the back or the legs in the absence of inflammation. It is purely a neurological loss. Radiculopathy may be associated with somatic referred pain or with radicular pain.[2]
Mechanism of Pain
There is extensive evidence that radicular pain requires neural traction in the presence of inflammation in disc herniation.[3]
Clinical Evidence: There is clinical evidence of an inflammatory component of pain
- Discectomy is an evidence based treatment, but is not universally successful despite technically adequate decompression
- The size of a disc herniation is not very well correlated with the presence and severity of pain.
- Large disc herniations may be completely asymptomatic
- There is little prognostic value in the imaging features of a disc herniation
- Conservative treatment is often effective
Experimental Evidence: There is also experimental evidence that suggests an inflammatory component.
The natural history of disc herniation is that of resorption. This occurs through metalloproteases that are produced by macrophages.
The nucleus pulposus is inflammatogenic and leucotatic. It induces the production of PLA2, interleukins (1α, 1β, 6), TNFα, and nitric oxide.
The experimental introduction of nucleus pulposis into the epidural space induces nerve dysfunction and degeneration. Nucleus pulposus in the epidural space causes a delay in nerve conduction velocity, however intravenous methylprednisolone 24 hours prior to this prevents this. Nucleus pulposus in the epidural space results in mechanical hyperalgesia which correlates with prostaglandin A2 immunoreactivity. Annulus fibrosus and nucleus pulposus in the epidural space results in thermal hyperalgesia which correlates with nitric oxide levels in the disc material, but mechanical hypoalgesia.
Radicular pain requires traction on a nerve root in the presence of inflammation. Traction alone only causes nerve dysfunction. Chronic compression of a nerve root can result in it becoming sensitised to mechanical stimuli. Possible mechanisms include partial damage to axons, neuroma-in-continuity, focal demyelination, intraneural oedema, and impaired microcirculation.
Acute mechanical compression of a dorsal root ganglion causes ectopic activity in Aδ, C and Aβ fibres. This multimodal activation causes a distinctive quality that is shooting or electric in nature. However acute compression of a nerve root only results in brief activity of nociceptive fibres. Radicular pain also requires an inflammatory reaction. If nucleus pulposus is applied to a dorsal root ganglion then there is discharge in Aδ and Aβ fibres.
Clinical Features
Radicular pain is distinct from somatic referred pain. Radicular pain is shooting and band-like. Somatic referred pain on the other hand is constant in position, poorly localised, diffuse, and aching in quality.[2]
Pain Pattern
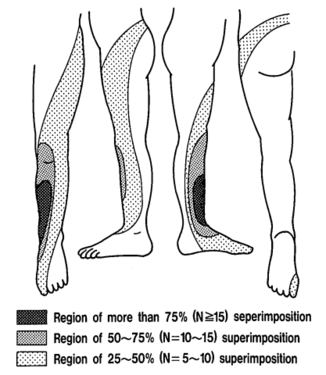
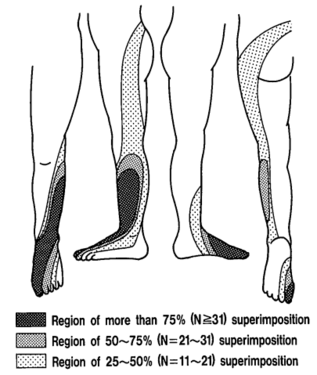
The pattern of lumbar radicular pain is not dermatomal, but rather dynatomal. Furman et al in 2019 mapped lower limb radicular symptoms based on inadvertent pain patterns during supraneural transforaminal injections, the data is modified in table format below. Unfortunately they did not use detailed grid based mapping like Slipman and colleagues did for the cervical spine. Furman found that pain patterns can't predict the nerve root level for the lumbar spine. The buttock is a very common pain referral location across all nerve roots, while pain in the thigh and leg frequently follows dermatomal distributions.[4]
| Nerve Root | L3 | L4 | L5 | S1 |
|---|---|---|---|---|
| Buttock | 45% | 43% | 62% | 64% |
| Groin | 0% | 3% | 0% | 0% |
| Anterior Thigh | 27% | 29% | 12% | 0% |
| Posterior Thigh | 36% | 25% | 59% | 36% |
| Medial Thigh | 18% | 11% | 3% | 0% |
| Lateral Thigh | 0% | 14% | 9% | 0% |
| Knee | 9% | 7% | 6% | 9% |
| Anterior Leg | 0% | 14% | 3% | 0% |
| Posterior Leg | 18% | 18% | 50% | 45% |
| Medial Leg | 0% | 7% | 6% | 0% |
| Lateral Leg | 9% | 14% | 24% | 0% |
| Foot | 0% | 3% | 0% | 0% |
Pain below the knee is not a helpful indicator of radicular pain as it can occur in other conditions such as somatic referred pain.[5] A careful history should be elicited to distinguish between somatic referred and radicular pain with regards to the pain quality, distribution, pattern, and depth. However they are not mutually exclusive, they can co-exist.
| Somatic Referred | Radicular | |
|---|---|---|
| Pain quality | Dull, deep ache, or pressure-like, perhaps like an expanding pressure | Shooting, lancinating, or electric-shocks |
| Relation to back pain | Referred pain is always concurrent with back pain. If the back pain ceases then so does the referred pain. If the back pain flares then so does the leg pain intensity and spatial spread. | Not always concurrent with back pain. |
| Distribution | Anywhere in the lower limb, fixed in location, commonly in the buttock or proximal thigh. Spread of pain distal to the knee can occur when severe even to the foot, and it can skip regions such as the thigh. It can feel like an expanding pressure into the lower limb, but remains in location once established without traveling. It can wax and wane, but does so in the same location. | Entire length of lower limb, but below knee > above knee. In mild cases the pain may be restricted proximally. |
| Pattern | Felt in a wide area, with difficult to perceive boundaries, often demonstrated with an open hand rather than pointing finger. The centres in contrast can be confidently indicated. | Travels along a narrow band no more than 5-8 cm wide in a quasi-segmental fashion but not related to dermatomes (dynatomal). |
| Depth | Deep only, lacks any cutaneous quality | Deep as well as superficial |
| Neurological signs | Not characteristic | Favours radicular pain, but not required. |
| Neuroanatomical basis | Discharge of the peripheral nerve endings of Aδ and C fibres from the lower back converge onto second order neurons in the dorsal horn that also receive input from from the lower limb, and so the frontal lobe has no way of knowing where the pain came from. | Heterotopic discharge of Aδ, Aβ, and C fibres through stimulation of a dorsal root or dorsal root ganglion of a spinal nerve, typically in the presence of inflammation, with pain being felt in the peripheral innervation of the affected nerve |
Examination
The neurological examination determines the presence of radiculopathy, but doesn't distinguish the cause of the radiculopathy.
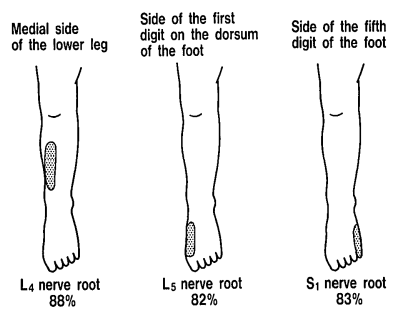
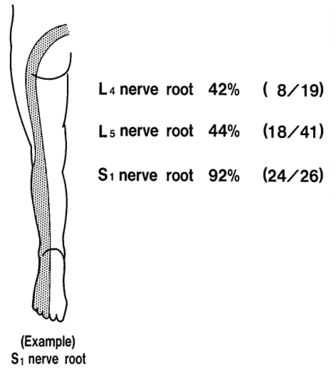
Sensory loss patterns were evaluated by Nitta and colleagues through fluoroscopically guided spinal nerve anaesthetic blocks in patients with radicular pain. They found the following sensory deficits.[7] This is not necessarily what typically occurs in patients not undergoing selective nerve root blocks, but simply the dermatomes for the nerve roots.
Hyporeflexia can be a useful finding. (See also Reflex Testing)[8]
- A loss of quadriceps reflex has a positive likelihood ratio of 8.5 for L3 or L4 radiculopathy
- A loss of hamstring reflex has a positive likelihood ratio of 6.2 for L5 radiculopathy
- A loss of Achilles reflex has a positive likelihood ratio of 2.7 for S1 radiculopathy
Weakness occurs in a myotomal distribution. Note in the complete myotome chart in the link that proximal muscles are supplied by some of the same nerves that are typically affected in disc prolapse. For example an L5 radiculopathy may not only cause a foot drop, but also gluteus medius weakness.
As part of a motor assessment one should also assess for wasting. Calf wasting is suggestive of an S1 radiculopathy.
Provocative signs can be tested which include the straight-leg raise test, crossed straight-leg raise test, and slump test. The pooled +LR of the straight leg raise test is only 1.3. The slump test may be better but it has not received as much study.[9] The femoral stretch test used for assessing high lumbar radicular pain has also not received as much study but may have some moderate utility.[10]
The centralisation phenomenon has been shown to be both highly reliable and valid in identifying painful lumbar discs.[11]
Trigger points in the superior-lateral quadrant of the gluteal area have a positive LR of 8.62 and negative LR of 0.28 for lumbar radicular pain.[12]
Differential Diagnosis
The neurological examination can also help to differentiate between radiculopathy, cauda equina syndrome, lumbosacral plexopathy, and peripheral nerve lesions.
Lumbosacral plexopathy: Unlikely with brachial plexus lesions where discrete upper and lower plexus syndromes can occur, lumbosacral plexopathies tend to affect the entire leg (L2-S1) simultaneously. The two classic situations where lumbosacral plexopathy occurs is in cancer patients and diabetics.
In cancer patients, if the findings only affect one leg then that suggests recurrence of their tumour. Findings in both legs is consistent with radiation plexopathy
In diabetics there is a condition called diabetic amyotrophy. It presents with proximal weakness and severe pain in the thighs, back, or both. The weakness may be unilateral or bilateral, but is always asymmetric. The quadriceps reflex can be helpful as it is absent in 80% of patients. Sensation is often normal.[8]
Peripheral nerve lesions: peripheral nerve lesions cause weakness in two or more muscles from a single peripheral nerve (which may belong to different spinal segments) and spare muscles from other nerves.
For example, over 85% of patients with foot drop secondary to peroneal nerve injury have weak ankle dorsiflexion (L4-L5) and eversion (L5-S1) but have preservation of inversion. The tibial nerve innervates tibialis posterior (L4-L5) but is spared in a peroneal nerve injury. So with L4-L5 working for inversion, but not working for ankle dorsiflexion and eversion, suggests that the lesion is not in the spine, but in the peroneal nerve.
Also, the peroneal nerve does not contribute to the Achilles reflex. So in patients with foot drop, an absent or asymmetrically diminished ankle jerk reduces the probability that they have a peroneal nerve palsy, and increases the chance that they have a more proximal lesion such as a sciatic neuropathy or radiculopathy..
Another important thing to remember is that the sciatic trunk divides into the peroneal and tibial nerves just above the knee. Therefore a lesion of the sciatic trunk (sciatic neuropathy) can affect any of the muscles of the sciatic trunk, peroneal nerve, and tibial nerve (see complete myotome chart).
If there is weakness predominantly affecting the proximal leg muscles then sciatic, peroneal, and tibial neuropathy are unlikely. Proximal weakness would suggest femoral or obturator neuropathy, lumbosacral plexopathy, radiculopathy, or myopathy.
Cauda equina syndrome is a red flag clinical entity with which the doctor should be familiar with and is discussed in detail in the linked article. Disc herniation at L4-L5 and L5-S1 are the most common causes
Bladder, bowel, or sexual dysfunction in combination with saddle or perineal sensory deficit is needed to make a clinical diagnosis. The patient may have back pain, lower limbs weakness, lower limb sensory loss, and abnormal lower limb reflexes.
Urinary symptoms include reduced urethral sensation while voiding, urinary retention, and overflow urinary incontinence. Urinary retention confers a poor prognosis and the diagnosis has been made too late (white flag).
Disc herniation if only centrally located, may not compress the lateral lumbar nerve roots, and so the patient may not have lower limb pain or neurological deficit.
Investigations
Electrophysiology Studies
Electrophysiological studies have low sensitivity and specificity and should not be used to confirm or refute the presence of a radiculopathy. Radicular pain also cannot be determined by such testing as it doesn't actually evaluate the neurologic mechanisms associated with pain generation. Pain is mediated by the small Aδ and C fibres, but examines effects of large fibres and as such is not a direct test of pain. Furthermore, in the presence of a radiculopathy with large fibre involvement, they cannot accurately determine the spinal nerve level.
MRI
Examination confirms the presence of radiculopathy, but imaging is required for definitive diagnosis of the cause of the radiculopathy.
Like all imaging modalities, MRI is operator dependent. For determining disc herniation versus non-herniation, interobserver agreement is quite good at 95%. However the reliability with regards to the descriptions and specific nature are not as good. For identifying and grading disc degeneration on MRI the intra-observer reliability is good (kappa = 0.7), however the inter-observer agreement is poor (kappa = 0.4 to 0.5). For classifying disc herniation the interobserver agreement is moderate (kappa = 0.58). [13][14]
The validity of MRI for the assessment of lumbar radicular pain is hindered by the high prevalence of disc abnormalities in asymptomatic individuals. The rate of herniated nucleus pulposus in one study of patients who had never had back pain was 24% for all ages. Disc bulges are so common that they should really be considered to be a normal feature.[15]
Furthermore, there is nothing about the size of a herniation that dictates whether the patient is symptomatic or not. In fact, large herniations reduce in size to a greater extent than small herniations and therefore tend to have a better prognosis.
Due to the low pre-test probability of finding a serious condition, MRI should not be used as a screening test for such conditions in the absence of red flags. It is best reserved for not with not responding to conservative treatment or for whom surgery is contemplated.
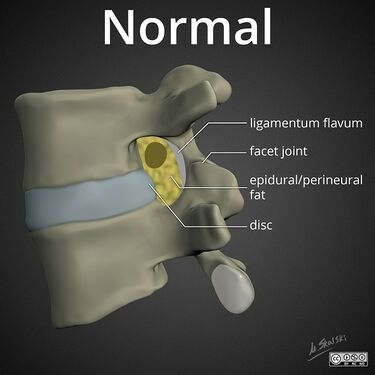
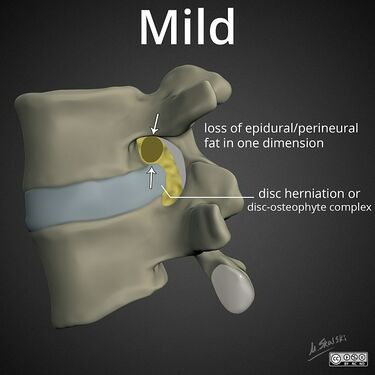
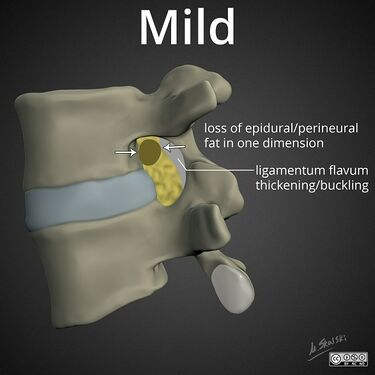
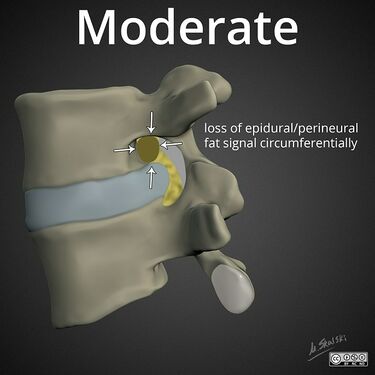
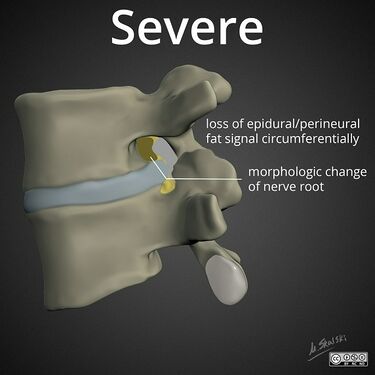
Lumbar nerve root compromise grading MRI - Pfirrmann 2004
Prognosis
The natural history of lumbar radicular pain was reported by Weber and colleagues in 1993. 214 patients were divided into two groups, one treated with placebo plus paracetamol plus bedrest, and the other treated with piroxicam plus paracetamol plus bedrest. In both groups, at four weeks 50% were free of leg pain, at 12 months 49% were free of leg pain (women worse prognosis with 33% free of leg pain). At 4 weeks and 12 months 60-70% had back pain. Essentially they found that 50% were free of leg pain long term, and chronic low back pain was very common.[16]
With more modern management, at 5 years 8% of patients never recover and 23% have ongoing complaints that fluctuate over time regardless of surgical versus non-surgical treatment. Unfavourable predictors were age over 40, severe leg pain at baseline, and higher affective McGill pain scores.[17]
Treatment
Injections
- Main article: Lumbar Transforaminal Epidural Steroid Injection
The Pain Physician Epidural Guidelines concluded that there is level I evidence for caudal epidural, lumbar interlaminar epidural, and lumbar transforaminal epidural injections[19]
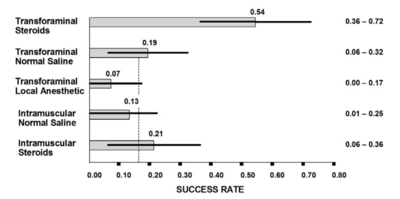
Some systematic reviews have used mean pain scores to show only modest benefit of epidural injections[20] but this is a misleading measure. More useful are categorical outcomes, i.e. what percentage responded. The systematic reviews also have tended to combine all types of epidurals.
There is good evidence that transforaminal epidural corticosteroid injections (TFIs) are effective[21][22][18], and safe[23] in the short term. Using categorical data, lumbar TFIs are effective in about 50% of patients.[18] Because half the treated sample responds well while the other half does not the mean scores misleadingly tells you that it isn't effective. There is no evidence supporting that epidural injections are effective in the long term, however epidural injections can be repeated.
5% dextrose is more effective than saline but the effect wanes at two weeks.[24]
Physical Therapy
A variety of approaches have been studied, for example the McKenzie Mechanical Diagnosis and Treatment approach, and the Mulligan approach.[25]
Surgery
Microdiscectomy is superior to nonoperative care (including epidural injection) if it is doing between 4-12 months for leg pain up to 12 months.[26] It is not generally recommended before 4 months, it may lead to more rapid relief of pain but the superiority is not sustained.[27][28] At 5 years, surgery is not more effective than prolonged conservative care, but recovery within the first year was better with early surgery. The trial was limited by a 46% crossover rate from conservative to surgical care.[17] Operating outside the strict inclusion criteria of trials may result in significantly less favourable outcomes as shown by a recent cohort study with an 8 year re-operation rate of 26% and an unfavourable recovery in 35%.[29]
There is little in the way of evidence to guide the decision for or against surgery in the setting of symptomatic lumbar spinal stenosis.[30]
A now old-fashioned treatment is chemonucleolysis (injecting chemicals to shrink the disc) which was proven to be affective in placebo controlled trials as an imperfect cure.[31] Microdiscectomy has replaced this treatment and so it isn't done in New Zealand.
See Also
- Cervical Radicular Pain
- Radicular Pain and Radiculopathy
- Neuropathic Pain
- Acute Low Back Pain
- Chronic Low Back Pain
- Lumbar Transforaminal Epidural Steroid Injection
- Caudal Epidural Steroid Injection
References
- ↑ Bogduk. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain 2009. 147:17-9. PMID: 19762151. DOI.
- ↑ 2.0 2.1 2.2 Bogduk, Nikolai. Clinical and radiological anatomy of the lumbar spine. Chapter 15. Edinburgh: Elsevier/Churchill Livingstone, 2012
- ↑ Mulleman D, Mammou S, Griffoul I, Watier H, Goupille P. Pathophysiology of disk-related sciatica. I.--Evidence supporting a chemical component. Joint Bone Spine. 2006 Mar;73(2):151-8. doi: 10.1016/j.jbspin.2005.03.003. Epub 2005 Jun 22. PMID: 16046173.
- ↑ Furman & Johnson. Induced lumbosacral radicular symptom referral patterns: a descriptive study. The spine journal : official journal of the North American Spine Society 2019. 19:163-170. PMID: 29800710. DOI.
- ↑ Haldeman S, Shouka M, Robboy S. Computed tomography, electrodiagnostic and clinical findings in chronic workers' compensation patients with back and leg pain. Spine (Phila Pa 1976). 1988 Mar;13(3):345-50. doi: 10.1097/00007632-198803000-00021. PMID: 2968667.
- ↑ Bogduk et al. Medical Management of Acute and Chronic Low Back Pain: An Evidence Based Approach. Elsevier Science. 2002
- ↑ Nitta et al.. Study on dermatomes by means of selective lumbar spinal nerve block. Spine 1993. 18:1782-6. PMID: 8235861. DOI.
- ↑ 8.0 8.1 McGee, Steven R. Evidence-based physical diagnosis. Philadelphia, PA: Elsevier, 2018.
- ↑ van der Windt DA, Simons E, Riphagen II, Ammendolia C, Verhagen AP, Laslett M, Devillé W, Deyo RA, Bouter LM, de Vet HC, Aertgeerts B. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. Cochrane Database Syst Rev. 2010 Feb 17;(2):CD007431. doi: 10.1002/14651858.CD007431.pub2. PMID: 20166095.
- ↑ Suri, Pradeep et al. “The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement.” Spine vol. 36,1 (2011): 63-73. doi:10.1097/BRS.0b013e3181c953cc
- ↑ Laslett M, Oberg B, Aprill CN, McDonald B. Centralization as a predictor of provocation discography results in chronic low back pain, and the influence of disability and distress on diagnostic power. Spine J. 2005 Jul-Aug;5(4):370-80. doi: 10.1016/j.spinee.2004.11.007. PMID: 15996606.
- ↑ Adelmanesh, Farhad; Jalali, Ali; Shirvani, Armin; Pakmanesh, Kambiz; Pourafkari, Marina; Raissi, Gholam R.; Shir, Yoram (2016-08). "The Diagnostic Accuracy of Gluteal Trigger Points to Differentiate Radicular From Nonradicular Low Back Pain". The Clinical Journal of Pain. 32 (8): 666–672. doi:10.1097/AJP.0000000000000311. ISSN 1536-5409. PMID 26491935. Check date values in:
|date=(help) - ↑ Raininko R, Manninen H, Battie MC, Gibbons LE, Gill KG, Fisher LD. Observer variability in the assessment of disc degeneration on magnetic resonance images of the lumbar and thoracic spine. spine 1995; 20:1029-1035.
- ↑ Brant –Zawadzki M, Jensen MC, Obuchowski N, Ross JS, Modic MT. Interobserver and intraobserver variability in interpretation of lumbar disc abnormalities. Spine 1995; 20:1257-1264.
- ↑ Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990 Mar;72(3):403-8. PMID: 2312537.
- ↑ Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine (Phila Pa 1976). 1993 Sep 1;18(11):1433-8. PMID: 8235813.
- ↑ 17.0 17.1 Lequin, Michiel B; Verbaan, Dagmar; Jacobs, Wilco C H; Brand, Ronald; Bouma, Gerrit J; Vandertop, William P; Peul, Wilco C (2013). "Surgery versus prolonged conservative treatment for sciatica: 5-year results of a randomised controlled trial". BMJ Open. 3 (5): e002534. doi:10.1136/bmjopen-2012-002534. ISSN 2044-6055.
- ↑ 18.0 18.1 18.2 Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Med. 2010 Aug;11(8):1149-68. doi: 10.1111/j.1526-4637.2010.00908.x. PMID: 20704666.
- ↑ Manchikanti L, et al. Epidural Interventions in the Management of Chronic Spinal Pain: American Society of Interventional Pain Physicians (ASIPP) Comprehensive Evidence-Based Guidelines. Pain Physician. 2021 Jan;24(S1):S27-S208. PMID: 33492918.
- ↑ Oliveira CB, Maher CG, Ferreira ML, Hancock MJ, Oliveira VC, McLachlan AJ, Koes BW, Ferreira PH, Cohen SP, Pinto RZ. Epidural corticosteroid injections for lumbosacral radicular pain. Cochrane Database Syst Rev. 2020 Apr 9;4(4):CD013577. doi: 10.1002/14651858.CD013577. PMID: 32271952; PMCID: PMC7145384.
- ↑ MacVicar et al.. The effectiveness of lumbar transforaminal injection of steroids: a comprehensive review with systematic analysis of the published data. Pain medicine (Malden, Mass.) 2013. 14:14-28. PMID: 23110347. DOI.
- ↑ Kaufmann et al.. Clinical effectiveness of single lumbar transforaminal epidural steroid injections. Pain medicine (Malden, Mass.) 2013. 14:1126-33. PMID: 23895182. DOI.
- ↑ El-Yahchouchi et al.. Adverse Event Rates Associated with Transforaminal and Interlaminar Epidural Steroid Injections: A Multi-Institutional Study. Pain medicine (Malden, Mass.) 2016. 17:239-49. PMID: 26593277. DOI.
- ↑ Maniquis-Smigel et al.. Short Term Analgesic Effects of 5% Dextrose Epidural Injections for Chronic Low Back Pain: A Randomized Controlled Trial. Anesthesiology and pain medicine 2017. 7:e42550. PMID: 28920043. DOI. Full Text.
- ↑ Danazumi MS, Bello B, Yakasai AM, Kaka B. Two manual therapy techniques for management of lumbar radiculopathy: a randomized clinical trial. J Osteopath Med. 2021 Feb 26;121(4):391-400. doi: 10.1515/jom-2020-0261. PMID: 33705612.
- ↑ Bailey, Chris S.; Rasoulinejad, Parham; Taylor, David; Sequeira, Keith; Miller, Thomas; Watson, Jim; Rosedale, Richard; Bailey, Stewart I.; Gurr, Kevin R.; Siddiqi, Fawaz; Glennie, Andrew (2020-03-19). "Surgery versus Conservative Care for Persistent Sciatica Lasting 4 to 12 Months". New England Journal of Medicine. 382 (12): 1093–1102. doi:10.1056/nejmoa1912658. ISSN 0028-4793.
- ↑ Peul WC, van den Hout WB, Brand R, Thomeer RT, Koes BW; Leiden-The Hague Spine Intervention Prognostic Study Group. Prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: two year results of a randomised controlled trial. BMJ. 2008 Jun 14;336(7657):1355-8. doi: 10.1136/bmj.a143. Epub 2008 May 23. PMID: 18502911; PMCID: PMC2427077.
- ↑ Osterman H, Seitsalo S, Karppinen J, Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine (Phila Pa 1976). 2006 Oct 1;31(21):2409-14. doi: 10.1097/01.brs.0000239178.08796.52. PMID: 17023847.
- ↑ Lequin, Michiel.B.; Verbaan, Dagmar; Schuurman, Peter R.; Tasche, Saskia; Peul, Wilco C.; Vandertop, William P.; Bouma, Gerrit J. (2022-01-07). "Microdiscectomy for sciatica: reality check study of long-term surgical treatment effects of a Lumbosacral radicular syndrome (LSRS)". European Spine Journal. 31 (2): 400–407. doi:10.1007/s00586-021-07074-x. ISSN 0940-6719.
- ↑ Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016 Jan 29;2016(1):CD010264. doi: 10.1002/14651858.CD010264.pub2. PMID: 26824399; PMCID: PMC6669253.
- ↑ Couto JM, Castilho EA, Menezes PR. Chemonucleolysis in lumbar disc herniation: a meta-analysis. Clinics (Sao Paulo). 2007 Apr;62(2):175-80. doi: 10.1590/s1807-59322007000200013. PMID: 17505703.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,