Myositis: Difference between revisions
(→MRI) |
|||
| Line 46: | Line 46: | ||
In myopathies, there is a drop-out of individual muscle fibres. Motor neurons and axons are not affected. In myopathies each motor unit action potential (MUAP) is generated by fewer motor fibres. | In myopathies, there is a drop-out of individual muscle fibres. Motor neurons and axons are not affected. In myopathies each motor unit action potential (MUAP) is generated by fewer motor fibres. | ||
Therefore with EMG you see polyphasic, shorter duration, and low amplitude results. This is how you determine that it is an intrinsic muscle process. ALS on the other hand will have normal EMG. In comparison, nerve conduction studies will be abnormal in ALS but normal in myopathies. | Therefore with EMG you see polyphasic, shorter duration, and low amplitude results. This is how you determine that it is an intrinsic muscle process. ALS on the other hand will have normal EMG. In comparison, nerve conduction studies will be abnormal in ALS but normal in myopathies. | ||
=== MRI === | === MRI === | ||
On T2 weighted images and STIR you see increased oedema signal. This doesn't diagnose myositis as such but it can tell you which muscle is involved and it can be helpful in planning muscle biopsy to get the best biopsy specimen This is important because in myositis the inflammation can be patchy. | On T2 weighted images and STIR you see increased oedema signal. This doesn't diagnose myositis as such but it can tell you which muscle is involved and it can be helpful in planning muscle biopsy to get the best biopsy specimen This is important because in myositis the inflammation can be patchy. | ||
| Line 54: | Line 53: | ||
=== Muscle biopsy === | === Muscle biopsy === | ||
=== Myositis Autoantibodies === | |||
{| class="wikitable" | |||
|+Diagnostic interpretation (from Labplus Dec 2022<ref>{{Cite web|last=|first=|date=|title=Myositis antibodies|url=https://testguide.adhb.govt.nz/EGuide/|url-status=live|archive-url=|archive-date=|access-date=2022-12-15|website=testguide.adhb.govt.nz}}</ref>) | |||
! '''Target''' | |||
! '''Myositis antibody Group''' | |||
! '''Clinical association''' | |||
! '''ANA association''' | |||
! '''Other comments''' | |||
|- | |||
|Mi-2 alpha | |||
Chromodomain-helicase-DNA binding protein 3 | |||
|MSA | |||
|DM (20%) | |||
|Nuclear fine speckled | |||
(AC-4) | |||
| | |||
|- | |||
|Mi-2 beta | |||
Chromodomain-helicase-DNA binding protein 4 | |||
|MSA | |||
|DM (20%) | |||
|Nuclear fine speckled | |||
(AC-4) | |||
|More frequently detected in adult onset DM patients | |||
|- | |||
|TIF 1 gamma | |||
Transcriptional intermediary factor -1 gamma | |||
|MSA | |||
|DM (15%) | |||
|Nuclear fine speckled | |||
(AC-4) | |||
|In 50-60% DM patients who are TIF 1 gamma antibody positive there is an association with malgnant disease (e.g. pancreatic carcinoma). The presence of TIF 1gamma in juvenile DM is not associated with malignancy | |||
|- | |||
|MDA 5 | |||
Melanoma differentiation ?associated gene 5 | |||
|MSA | |||
|DM (all 15-25%) | |||
>95% of patients with amyopathic DM | |||
|Negative or cytoplasmic based fine speckling | |||
(AC-20) | |||
|Amyopathic DM - prominent skin changes with only minimal muscle invovement. There are systemic features with rapidly progressive ILD | |||
|- | |||
|NXP 2 | |||
RNA and nuclear matrix binding protein 2 | |||
|MSA | |||
|Juvenile PM / DM (20-30%) | |||
Adult PM / DM (1%) | |||
|Multiple nuclear dots | |||
(AC-6) | |||
|Associated with juvenile DM. Clinical features may include severe muscle weakness, calcinosis, joiny contractures, intestinal vasculitis. | |||
Adult cases may be carcinoma associated (breast, uterine, pancreatic) | |||
|- | |||
|SAE 1 | |||
SUMO activating enzyme subunits 1 | |||
|MSA | |||
|DM (8%) | |||
|Nuclear fine speckled | |||
(AC-4) | |||
|In adult DM, 5% cases have ILD. Cutaneous features may precede muscle involvement. | |||
|- | |||
|SRP | |||
Signal recognition particle | |||
|MSA | |||
|IMMN | |||
(anti-SRP syndrome) | |||
|Cytoplasmic based dense fine speckling | |||
(AC-19) | |||
| | |||
|- | |||
|Jo-1 | |||
Histidyl-tRNA synthetase | |||
|MSA | |||
|PM (20-60%) | |||
|Cytoplasmic fine speckled (variable expression) | |||
(AC-20) | |||
|Seen in patients with synthetase syndrome | |||
Frequently associated with ILD | |||
If Jo-1 is specific, expect to have Ro52 reactivity | |||
|- | |||
|PL-7 | |||
Threonyl-tRNA synthetase | |||
|MSA | |||
|Myositis (5-10%) | |||
|Cytoplasmic fine speckled (variable expression) | |||
(AC-20) | |||
|Seen in patients with synthetase syndrome | |||
|- | |||
|PL-12 | |||
Alanyl-tRNA synthetase | |||
|MSA | |||
|Myositis (5%) | |||
|Cytoplasmic fine speckled (variable expression) | |||
(AC-20) | |||
|Seen in patients with synthetase syndrome | |||
|- | |||
|EJ | |||
Glycyl-tRNA synthetase | |||
|MSA | |||
|PM (1-5%) | |||
|Cytoplasmic fine speckled (variable expression) | |||
(AC-20) | |||
|Seen in patients with synthetase syndrome | |||
|- | |||
|OJ | |||
Isoleucyl-tRNA synthetase | |||
|MSA | |||
|PM (1-5%) | |||
|Cytoplasmic fine speckled (variable expression) | |||
(AC-20) | |||
|Seen in patients with synthetase syndrome | |||
|- | |||
|NT5c1A cN-1A | |||
Cytosolic 5'-nucleotidase 1A | |||
|MSA | |||
|sIBM (35%) | |||
PM (75%) | |||
DM (15%) | |||
|Unknown | |||
|While considered a marker for sporadic inclusion body myositis, is also seen in other CTD's and 5% of healthy individuals | |||
|- | |||
|HMGCR | |||
3-hydroxy-3-methylglutaryl coenzyme A reductase | |||
|MSA | |||
|Necrotising myositis (75%) | |||
|Negative or | |||
Cytoplasmic based speckling | |||
(AC-20) | |||
|Most but not all cases are related to statin use | |||
|- | |||
|Ku | |||
DNA binding, non-histone protein | |||
|MAA | |||
|Seen in idiopathic inflammatory myopathy | |||
|Nuclear fine speckled | |||
(AC-4) | |||
|Associated with increased frequency of vasculitis and pulmonary hypertension. | |||
Seen in other CTD's notably SLE and Systemic sclerosis. | |||
|- | |||
|PM-ScL | |||
Proteins (75 kDa and 100 kDa) of the nucleolar PM-ScL macromolecular complex PM-1) | |||
|MAA | |||
|Paients with PM / DM and or SSc overlap syndrome | |||
(50-70%) | |||
|Nuclear: Homogeneous and Nucleolar pattern | |||
(AC-8) | |||
|The two proteins of differing molecular weights (75kDa and 100kDa) are not separately reported. If either is reactive, the target is reported as positive. | |||
|- | |||
|Ro52 | |||
Zinc binding protein as a member of the tripartite motif (TRIM) family | |||
|MAA | |||
|Myositis patients (25%) | |||
|No specific ANA pattern is associated with this target | |||
|} | |||
== Resources == | == Resources == | ||
Revision as of 16:55, 15 December 2022
Myositis is a group of rare autoimmune diseases that cause inflammation of the skeletal muscles. Myositis can be difficult to diagnose due to its varied and sometimes nonspecific symptoms
Classification
The original Bohan and Peter classification which includes 5 groups from 1975 has been the most widely used classification criteria. The criteria are sensitive but not specific for diagnosing myositis.Over the years there has been increasing recognition of involvement of other organ systems such as interstitial lung disease. In 2017 EULAR-ACR developed a new classification criteria. These new criteria are more specific.
There are four main subtypes of myositis: dermatomyositis, polymyositis, immune-mediated necrotising myopathy, and inclusion body myositis.
The clinical subclassification schema is limited by overlapping clinical and histopathological features. Subgrouping by myositis-specific autoantibodies is a newer classification method to overcome this. These autoantibodies are very specific and can help diagnose myositis. The autoantibodies are associated with distinct clinical features, including arthritis, lung disease, and skin manifestations.
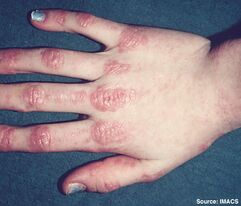
Dermatomyositis
This is the easiest subtype to diagnose due to the skin manifestations. Gottron's papules are found over the dorsal aspects of the MCPJs and PIPJs and even over the DIPJs. These can be itchy. The patient may have a very photosensitive V-neck distribution rash, very similar to lupus.
The muscle weakness pattern is proximal. including the neck, arm abductors, and hip flexors. On muscle biopsy there is peri-fascicular atrophy.
Immune Mediated Necrotising Myopathy
This was first described in 2010. These patients have the same pattern of weakness as dermatomyositis. However on biopsy there is necrosis of the muscle fibres. The myofibres are different in size which indicates a true intrinsic myopathic process. With treatment the fibres regenerate.
The important question is whether they have had statin exposure which has a strong link to autoimmune necrotising myositis. It is an extremely rare adverse event.
Polymyositis
This is a diagnosis of exclusion. On biopsy you should see primary inflammation , i.e. inflammatory cells invading the muscle fibres.
The polymyositis category is controversial as to whether it actually exists as many of these patients evolve to develop more typical features of another subtype or actually have a genetic neuromuscular disease such as facioscapulohumeral dystrophy or limb girdle muscular dystrophy.
Inclusion Body Myositis
Inclusion body myositis (IBM) is a chronic, inflammatory myopathy that primarily affects older adults, particularly men. The condition is characterized by proximal muscle weakness and the presence of inflammatory cells surrounding and invading myofibres and rimmed vacuoles on biopsy. IBM typically presents with asymmetric weakness of the proximal and distal limbs. Initial symptoms are often difficulty climbing stairs and rising from a seated position. In advanced cases, dysphagia and respiratory muscle weakness may also be observed.
The pathogenesis of IBM is not fully understood, but it is thought to be autoimmune. Overall, the prognosis for patients with IBM is poor, by 15 years most patients require assistance with basic daily activities.
Unfortunately the term IBM also stands for an unrelated category of diseases called the inclusion body myopathies. The myopathies are inherited muscle disorders (h-IBM), while the other type are autoimmune. The two different "IBMs" only have superficial resemblence.
Evaluation
The most important aspects for evaluation are:
- Pattern of muscle weakness, i.e. proximal or distal. IBM also has distal weakness
- Muscle enzyme elevation (CK)
- Muscle biopsy
- MRI of the muscles
- Neurophysiology studies
- Autoantibody myositis
Neurophysiology
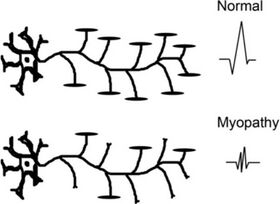
In myopathies, there is a drop-out of individual muscle fibres. Motor neurons and axons are not affected. In myopathies each motor unit action potential (MUAP) is generated by fewer motor fibres.
Therefore with EMG you see polyphasic, shorter duration, and low amplitude results. This is how you determine that it is an intrinsic muscle process. ALS on the other hand will have normal EMG. In comparison, nerve conduction studies will be abnormal in ALS but normal in myopathies.
MRI
On T2 weighted images and STIR you see increased oedema signal. This doesn't diagnose myositis as such but it can tell you which muscle is involved and it can be helpful in planning muscle biopsy to get the best biopsy specimen This is important because in myositis the inflammation can be patchy.
In chronic cases, such as in untreated myositis or in genetic muscular dystrophies where there is no effective treatment, there is increased signal on T1 indicating fatty atrophy and irreversible damage.
Muscle biopsy
Myositis Autoantibodies
| Target | Myositis antibody Group | Clinical association | ANA association | Other comments |
|---|---|---|---|---|
| Mi-2 alpha
Chromodomain-helicase-DNA binding protein 3 |
MSA | DM (20%) | Nuclear fine speckled
(AC-4) |
|
| Mi-2 beta
Chromodomain-helicase-DNA binding protein 4 |
MSA | DM (20%) | Nuclear fine speckled
(AC-4) |
More frequently detected in adult onset DM patients |
| TIF 1 gamma
Transcriptional intermediary factor -1 gamma |
MSA | DM (15%) | Nuclear fine speckled
(AC-4) |
In 50-60% DM patients who are TIF 1 gamma antibody positive there is an association with malgnant disease (e.g. pancreatic carcinoma). The presence of TIF 1gamma in juvenile DM is not associated with malignancy |
| MDA 5
Melanoma differentiation ?associated gene 5 |
MSA | DM (all 15-25%)
>95% of patients with amyopathic DM |
Negative or cytoplasmic based fine speckling
(AC-20) |
Amyopathic DM - prominent skin changes with only minimal muscle invovement. There are systemic features with rapidly progressive ILD |
| NXP 2
RNA and nuclear matrix binding protein 2 |
MSA | Juvenile PM / DM (20-30%)
Adult PM / DM (1%) |
Multiple nuclear dots
(AC-6) |
Associated with juvenile DM. Clinical features may include severe muscle weakness, calcinosis, joiny contractures, intestinal vasculitis.
Adult cases may be carcinoma associated (breast, uterine, pancreatic) |
| SAE 1
SUMO activating enzyme subunits 1 |
MSA | DM (8%) | Nuclear fine speckled
(AC-4) |
In adult DM, 5% cases have ILD. Cutaneous features may precede muscle involvement. |
| SRP
Signal recognition particle |
MSA | IMMN
(anti-SRP syndrome) |
Cytoplasmic based dense fine speckling
(AC-19) |
|
| Jo-1
Histidyl-tRNA synthetase |
MSA | PM (20-60%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome
Frequently associated with ILD If Jo-1 is specific, expect to have Ro52 reactivity |
| PL-7
Threonyl-tRNA synthetase |
MSA | Myositis (5-10%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome |
| PL-12
Alanyl-tRNA synthetase |
MSA | Myositis (5%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome |
| EJ
Glycyl-tRNA synthetase |
MSA | PM (1-5%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome |
| OJ
Isoleucyl-tRNA synthetase |
MSA | PM (1-5%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome |
| NT5c1A cN-1A
Cytosolic 5'-nucleotidase 1A |
MSA | sIBM (35%)
PM (75%) DM (15%) |
Unknown | While considered a marker for sporadic inclusion body myositis, is also seen in other CTD's and 5% of healthy individuals |
| HMGCR
3-hydroxy-3-methylglutaryl coenzyme A reductase |
MSA | Necrotising myositis (75%) | Negative or
Cytoplasmic based speckling (AC-20) |
Most but not all cases are related to statin use |
| Ku
DNA binding, non-histone protein |
MAA | Seen in idiopathic inflammatory myopathy | Nuclear fine speckled
(AC-4) |
Associated with increased frequency of vasculitis and pulmonary hypertension.
Seen in other CTD's notably SLE and Systemic sclerosis. |
| PM-ScL
Proteins (75 kDa and 100 kDa) of the nucleolar PM-ScL macromolecular complex PM-1) |
MAA | Paients with PM / DM and or SSc overlap syndrome
(50-70%) |
Nuclear: Homogeneous and Nucleolar pattern
(AC-8) |
The two proteins of differing molecular weights (75kDa and 100kDa) are not separately reported. If either is reactive, the target is reported as positive. |
| Ro52
Zinc binding protein as a member of the tripartite motif (TRIM) family |
MAA | Myositis patients (25%) | No specific ANA pattern is associated with this target |
Resources
https://www.youtube.com/watch?v=sz5AVWo-Suo
References
- ↑ Paganoni, Sabrina; Amato, Anthony (2013-02). "Electrodiagnostic evaluation of myopathies". Physical Medicine and Rehabilitation Clinics of North America. 24 (1): 193–207. doi:10.1016/j.pmr.2012.08.017. ISSN 1558-1381. PMC 4435557. PMID 23177039. Check date values in:
|date=(help) - ↑ "Myositis antibodies". testguide.adhb.govt.nz. Retrieved 2022-12-15.