Myositis
Myositis, also known as idiopathic inflammatory myopathy, is a group of rare autoimmune diseases that cause inflammation of the skeletal muscles. Myositis can be difficult to diagnose due to its varied and sometimes nonspecific symptoms. Polymyalgia rheumatica and fibromyalgia are distinct to myositis in that they are not muscle diseases as such and muscle pain rather than true weakness is their predominant symptom.
Epidemiology
The incidence is approximately 1-6 per 100,000. There is a 2:1 female to male prevalence. Myositis can occur in all ages. In 20% there is an association with other autoimmune diseases, called overlap myositis, especially RA, SLE, and MCTD. In the elderly, dermatomyositis and polymyositis are often paraneoplastic, and symptoms can occur before tumour detection.
Classification
The original Bohan and Peter classification which includes 5 groups from 1975 has been the most widely used classification criteria. The criteria are sensitive but not specific for diagnosing myositis.Over the years there has been increasing recognition of involvement of other organ systems such as interstitial lung disease. In 2017 EULAR-ACR developed a new classification criteria. These new criteria are more specific.
There are four main subtypes of myositis: dermatomyositis, polymyositis, immune-mediated necrotising myopathy, and inclusion body myositis.
The clinical subclassification schema is limited by overlapping clinical and histopathological features. Subgrouping by myositis-specific autoantibodies is a newer classification method to overcome this. These autoantibodies are very specific and can help diagnose myositis. The autoantibodies are associated with distinct clinical features, including arthritis, lung disease, and skin manifestations.
Dermatomyositis
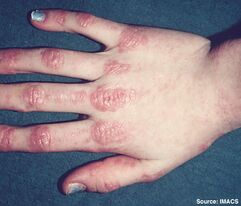
This is the easiest subtype to diagnose due to the skin manifestations. Gottron's papules are found over the dorsal aspects of the MCPJs and PIPJs and even over the DIPJs. These can be itchy. The patient may have a very photosensitive V-neck distribution rash, very similar to lupus.
The muscle weakness pattern is proximal. including the neck, arm abductors, and hip flexors. On muscle biopsy there is peri-fascicular atrophy.
Immune Mediated Necrotising Myopathy
This was first described in 2010. These patients have the same pattern of weakness as dermatomyositis. However on biopsy there is necrosis of the muscle fibres. The myofibres are different in size which indicates a true intrinsic myopathic process. With treatment the fibres regenerate.
The important question is whether they have had statin exposure which has a strong link to autoimmune necrotising myositis. It is an extremely rare adverse event. It has also been described as a post COVID event.[1]
Polymyositis
This is a diagnosis of exclusion. On biopsy you should see primary inflammation , i.e. inflammatory cells invading the muscle fibres.
The polymyositis category is controversial as to whether it actually exists as many of these patients evolve to develop more typical features of another subtype or actually have a genetic neuromuscular disease such as facioscapulohumeral dystrophy or limb girdle muscular dystrophy.
Inclusion Body Myositis
Inclusion body myositis (IBM) is a chronic, inflammatory myopathy that primarily affects older adults, particularly men. The condition is characterized by proximal muscle weakness and the presence of inflammatory cells surrounding and invading myofibres and rimmed vacuoles on biopsy. IBM typically presents with asymmetric weakness of the proximal and distal limbs. Initial symptoms are often difficulty climbing stairs and rising from a seated position. In advanced cases, dysphagia and respiratory muscle weakness may also be observed.
The pathogenesis of IBM is not fully understood, but it is thought to be autoimmune. Overall, the prognosis for patients with IBM is poor, by 15 years most patients require assistance with basic daily activities.
Unfortunately the term IBM also stands for an unrelated category of diseases called the inclusion body myopathies. The myopathies are inherited muscle disorders (h-IBM), while the other type are autoimmune. The two different "IBMs" only have superficial resemblence.
Antisynthetase Syndrome
While there are four main subtypes of myositis, some clinicians consider antisynthetase to be a distinct entity. The classic triad is myositis, interstitial lung disease, plus polyarthritis - along with positive antibodies. Other clinical features include "Mechanic's hands," fever, and Raynaud phenomenon.
Evaluation
The most important aspects for evaluation are:
- Pattern of muscle weakness, i.e. proximal or distal. IBM also has distal weakness
- Muscle enzyme elevation (CK)
- Neurophysiology studies
- MRI of the muscles
- Muscle biopsy
- Autoantibody myositis
Clinical Assessment
Other than assessment of limb strength, it is also important to assess neck flexor strength. Put the patient in the supine position and test neck flexion strength against resistance. This muscle group is often the first affected. For the limbs the classic pattern is arm abduction and hip flexion weakness.
Muscle Enzymes
Autoimmune myopathy is often accompanied by elevated levels of muscle enzymes in the blood, such as creatine kinase, aldolase, aspartate transaminase, and alanine transaminase. In over 90% of cases, patients with this condition have elevated levels of these enzymes. While high levels of creatine kinase are considered the most reliable indicator of muscle damage in these patients, it is possible for aldolase levels to be elevated without a corresponding increase in creatine kinase.[2] Aldolase is no longer offered at LabPlus in Auckland.
Neurophysiology
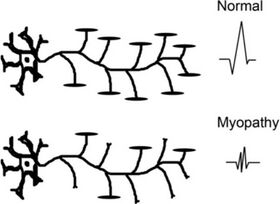
In myopathies, there is a drop-out of individual muscle fibres. Motor neurons and axons are not affected. In myopathies each motor unit action potential (MUAP) is generated by fewer motor fibres.
Therefore with EMG you see polyphasic, shorter duration, and low amplitude results. This is how you determine that it is an intrinsic muscle process. ALS on the other hand will have normal EMG. In comparison, nerve conduction studies will be abnormal in ALS but normal in myopathies.
MRI
On T2 weighted images and STIR you see increased oedema signal. This doesn't diagnose myositis as such but it can tell you which muscle is involved and it can be helpful in planning muscle biopsy to get the best biopsy specimen. This is important because in myositis the inflammation can be patchy and so muscle guided biopsy probably reduces the chance of false negatives.
In chronic cases, such as in untreated myositis or in genetic muscular dystrophies where there is no effective treatment, there is increased signal on T1 indicating fatty atrophy and irreversible damage.
Muscle biopsy
Muscle biopsies can provide valuable diagnostic information in patients with suspected autoimmune muscle disease. Bohan and Peter identified several features that are commonly associated with autoimmune myopathies, including degenerating and/or necrotic myofibers, regenerating muscle fibers, atrophic muscle cells, and evidence of inflammatory exudates. However, these features are not specific to autoimmune myopathies and can also be found in other conditions such as IBM and inflammatory muscular dystrophies.
Since Bohan and Peter published their classification scheme, researchers have identified unique pathological features in biopsies from patients with dermatomyositis, polymyositis, and IMNM. These features suggest that different underlying mechanisms are at play in each of these diseases. For example, evidence of atrophic, degenerating or regenerating fibers within the perifascicular area is considered pathognomonic for dermatomyositis. In contrast, biopsies from patients with polymyositis are characterized by the presence of cytotoxic T cells surrounding and invading non-necrotic myofibers. Biopsies from patients with IMNM typically show marked myofiber necrosis, degeneration and regeneration, with few, if any, inflammatory cells.[2]
Myositis Autoantibodies
The presence of myositis-specific autoantibodies (MSAs) can help differentiate between immune-mediated and non-immune-mediated muscle diseases. However, it is important to consider alternative diagnoses when specific features of dermatomyositis, polymyositis, or IMNM are absent, and to take into account features that are characteristic of other muscle diseases.
| Target | Myositis antibody Group | Clinical association | ANA association | Other comments |
|---|---|---|---|---|
| Mi-2 alpha | MSA | DM (20%) | Nuclear fine speckled
(AC-4) |
|
| Mi-2 beta | MSA | DM (20%) | Nuclear fine speckled
(AC-4) |
More frequently detected in adult onset DM patients |
| TIF 1 gamma | MSA | DM (15%) | Nuclear fine speckled
(AC-4) |
In 50-60% DM patients who are TIF 1 gamma antibody positive there is an association with malignant disease (e.g. pancreatic carcinoma). The presence of TIF 1 gamma in juvenile DM is not associated with malignancy |
| MDA 5 | MSA | DM (all 15-25%)
>95% of patients with amyopathic DM |
Negative or cytoplasmic based fine speckling
(AC-20) |
Amyopathic DM - prominent skin changes with only minimal muscle invovement. There are systemic features with rapidly progressive ILD |
| NXP 2 | MSA | Juvenile PM / DM (20-30%)
Adult PM / DM (1%) |
Multiple nuclear dots
(AC-6) |
Associated with juvenile DM. Clinical features may include severe muscle weakness, calcinosis, joiny contractures, intestinal vasculitis.
Adult cases may be carcinoma associated (breast, uterine, pancreatic) |
| SAE 1 | MSA | DM (8%) | Nuclear fine speckled
(AC-4) |
In adult DM, 5% cases have ILD. Cutaneous features may precede muscle involvement. |
| SRP | MSA | IMMN
(anti-SRP syndrome) |
Cytoplasmic based dense fine speckling
(AC-19) |
|
| Jo-1 | MSA | PM (20-60%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome
Frequently associated with ILD If Jo-1 is specific, expect to have Ro52 reactivity |
| PL-7 | MSA | Myositis (5-10%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome |
| PL-12 | MSA | Myositis (5%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome |
| EJ | MSA | PM (1-5%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome |
| OJ | MSA | PM (1-5%) | Cytoplasmic fine speckled (variable expression)
(AC-20) |
Seen in patients with synthetase syndrome |
| NT5c1A | MSA | sIBM (35%)
PM (75%) DM (15%) |
Unknown | While considered a marker for sporadic inclusion body myositis, is also seen in other CTD's and 5% of healthy individuals |
| HMGCR | MSA | Necrotising myositis (75%) | Negative or
Cytoplasmic based speckling (AC-20) |
Most but not all cases are related to statin use |
| Ku | MAA | Seen in idiopathic inflammatory myopathy | Nuclear fine speckled
(AC-4) |
Associated with increased frequency of vasculitis and pulmonary hypertension.
Seen in other CTD's notably SLE and Systemic sclerosis. |
| PM-ScL | MAA | Paients with PM / DM and or SSc overlap syndrome
(50-70%) |
Nuclear: Homogeneous and Nucleolar pattern
(AC-8) |
The two proteins of differing molecular weights (75kDa and 100kDa) are not separately reported. If either is reactive, the target is reported as positive. |
| Ro52 | MAA | Myositis patients (25%) | No specific ANA pattern is associated with this target |
Treatment
Glucocorticoids are started initially at 1mg/kg for prednisone. Either at the same time or soon afterwards another agent is started. The agents usually used are methotrexate, azathioprine, or mycophenolate. There are no studies comparing agents. Combination therapy is common. Other options include IVIG and rituximab.
Resources
https://www.youtube.com/watch?v=sz5AVWo-Suo
References
- ↑ Veyseh, Maedeh; Koyoda, Sai; Ayesha, Bibi (2021-04-13). "COVID-19 IgG-related autoimmune inflammatory necrotizing myositis". BMJ case reports. 14 (4): e239457. doi:10.1136/bcr-2020-239457. ISSN 1757-790X. PMC 8051403. PMID 33849864.
- ↑ 2.0 2.1 Mammen, Andrew L. (2011-06-08). "Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis". Nature Reviews. Neurology. 7 (6): 343–354. doi:10.1038/nrneurol.2011.63. ISSN 1759-4766. PMID 21654717.
- ↑ Paganoni, Sabrina; Amato, Anthony (2013-02). "Electrodiagnostic evaluation of myopathies". Physical Medicine and Rehabilitation Clinics of North America. 24 (1): 193–207. doi:10.1016/j.pmr.2012.08.017. ISSN 1558-1381. PMC 4435557. PMID 23177039. Check date values in:
|date=(help) - ↑ "Myositis antibodies". testguide.adhb.govt.nz. Retrieved 2022-12-15.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,