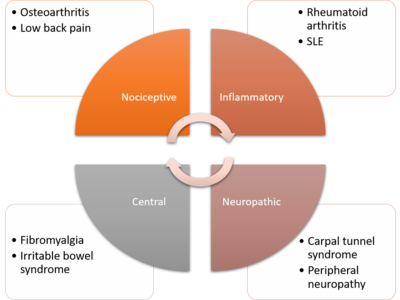
Nociplastic Pain
Musculoskeletal conditions can cause not only localised pain as a direct result from the condition, but also chronic widespread pain. This phenomenon has many terms with subtle differences in meaning, including central sensitisation, and nociplastic pain.[1]
Central sensitisation is found in 10 to 40 percent of those with osteoarthritis, rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, and systemic lupus erythematosus. It is also common in chronic trauma-induced low back and neck pain, complex regional pain syndrome, joint hypermobility syndrome, lateral elbow tendinopathy, and carpal tunnel syndrome. It is also thought to be a pain mechanism in fibromyalgia and other related chronic pain conditions, such as irritable bowel syndrome, bladder pain syndrome, and temporomandibular dysfunction.[1]
Epidemiology
The prevalence of chronic pain in New Zealand, when defined as lasting for 6 months or longer, was measured at 16.9% in 2011. Prevalence increased with age and economic deprivation. Pacific and Asian peoples had lower rates of chronic pain than European/other.[2] Around one fifth of people with chronic pain have predominantly neuropathic pain.[citation needed] Neuropathic pain is more disabling than other forms of pain and is associated with a lower quality of life.[citation needed]
Terminology
The IASP definitions of pain can be found on their website.
- IASP Definition of Pain
The IASP definition of pain was recently updated in 2020.[3]
“An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”
—IASP Definition of Pain 2020
- Sensitisation
Increased responsiveness of nociceptive neurons to their normal input, and/or recruitment of a response to normally subthreshold inputs...a neurophysiological term, may only be inferred indirectly from phenomena such as hyperalgesia or allodynia.
Spinal sensitisation is an increase in excitability and spontaneous discharge of the neurons of the dorsal horns of the spinal cord, an expansion of receptor fields, and an increase in responses evoked by the stimulation of small-calibre fibres (hyperalgesia) and large-calibre fibres (allodynia)
- Central Sensitisation
Woolf discussed the differences between pain versus pathology versus "pain syndromes."[4] The central component of post-injury pain hypersensitivity was first termed sensitisation in 1987.[5]
“Any sensory experience greater in amplitude, duration and spatial extent than that would be expected from a defined peripheral input under normal circumstances qualifies as potentially reflecting a central amplification due to increased excitation or reduced inhibition. These changes could include a reduction in threshold, exaggerated response to a noxious stimulus, pain after the end of a stimulus, and a spread of sensitivity to normal tissue”
—Woolf 2011
The IASP define central sensitisation as follows:
“Increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input.”
—IASP
- Peripheral sensitisation
Increase Responsiveness and reduced threshold of nociceptive neurons in the periphery to the stimulation of their receptive fields
- Nociceptive Pain
actual or threatened damage , non-neural tissue, activation of nociceptors.
- Neuropathic pain
Primary lesion or disease of the somatosensory nervous system.
Temporal Summation and Spatial Summation
Temporal and spatial summation reflect both active endogenous excitatory and inhibitory central mechanisms and are simple experimental paradigms for looking at the role of endogenous mechanisms in certain chronic pain states. Deficits in the inhibitory system is not characteristic of all chronic pain states, for example it is present in fibromyalgia but not chronic low back pain.[6]
Temporal Summation and Windup
High frequency repeated nociceptive stimulation produces a temporal summation of the nociceptive afferent impulses originating from the C fibres due to their relatively slow conduction velocities. This results in an increase in the perception of second pain without lessening the sensation of first pain, which is conducted rapidly by Aδ fibres. The accumulation of nociceptive activity within secondary neurons of the spinal cord is called windup. Windup is distinct from spinal sensitisation. Wind-up is an increase in frequency of C-fibre discharge. It is a transient phenomenon (thought to last minutes) but can produce long term potentiation/spinal sensitisation and become persistent.
Spatial Summation
With stimulation of a larger area of skin there is stimulation of a larger number of nociceptors. This multiplies the afferent impulses to the central nervous system. A stimulation of the same intensity is then perceived as being more intense on a large surface than on a smaller surface. The phenomenon of spatial summation involves both excitatory and inhibitory mechanisms.
Pain Mechanisms
Some patients with fibromyalgia actually have unrecognised small-fibre polyneuropathy (SFPN). Oaklander et al found SFPN in 41% of skin biopsies of patients diagnosed with fibromyalgia, compared to 3% of controls. This is a disease that causes dysfunction and degeneration of peripheral small-fibre neurons, and is a biologically plausible cause for chronic widespread pain. [7]
Anatomical and Functional Changes
Clinical Assessment
Examination Findings
There is no specific clinical test for central sensitisation. Clinical suspicion may be raised with persistent, spontaneous, and widespread pain, or with severe and prolonged pain following a seemingly innocuous stimulus.[8]
- Quantitative Sensory Testing
A surrogate marker is quantitative sensory testing (QST). QST evaluates sensitivity to a variety of stimuli with respect to heightened responsiveness. Secondary hyperalgesia (increased sensitivity to pain in normal tissue away from the painful site) can be evaluated by applying a nociceptive stimulus to an area that is innervated by a different segmental level. Tertiary hyperalgesia (increased sensitivity to pain on the contralateral side to the painful site) has been described in several conditions (e.g. knee osteoarthritis), and so the contralateral side to the painful site may not be a good control. QST values are best compared to normal reference values if available. QST is a surrogate measure, as it does not evaluate spontaneous pain, and only assesses superficial sensitivity rather than deep pain. See figure for clinical correlates.
- Dynamic Mechanical Allodynia
Dynamic mechanical allodynia is assessed by brushing the skin. Allodynia can be a sign of central sensitisation but can also be caused by peripheral drivers.
- Temporal Summation
This is examined by applying a repeated nociceptive stimulus (e.g. sharpness) within the same area. An increasing response is positive.
- Spatial Summation
This refers to an increased pain intensity with stimulation of increasingly wide areas, such as with pin prick.
- Conditioned Pain Modulation
Refers to one noxious stimulus inhibiting another. This is difficult to interpret.
Questionnaires
Essentially none of the various questionnaires are helpful for diagnosing nociplastic pain. The Central Sensitization Inventory and Pain Sensitivity Questionnaire have questionable construct validity and their namesakes are very misleading. They are not associated with widespread pain sensitivity, and so should not be used as such. They are more correlated with resilience, anxiety, and negative affect.[9] A review of 66 studies of over 13 thousand patients found that the CSI correlated with psychological hypervigilence rather than nociceptive sensitivity.[10] It is important to remember that psychological distress is not synonymous with central sensitisation. Chronic pain can lead to secondary psychological distress (the distress of which can resolve if the pain resolves for example in successful radiofrequency neurotomy with whiplash injuries), and psychological distress can influence central sensitisation through descending modulation.[8]
Diagnosis
For several reasons, the clinician should be extremely careful with diagnosing "central sensitisation syndrome." There are no agreed diagnostic criteria for detecting central sensitisation. Central sensitisation refers to a physiological process, and is not a diagnosis in itself. There are no highly validated tools for detecting this with questionnaires simply detecting psychological distress rather than pain sensitivity, and various examination findings having important limitations. It is important to not conflate psychological distress with central sensitisation. Peripheral pain drivers should always be considered as these can initiation, modulate, and maintain central sensitisation. Central processes can resolve with removal of a peripheral driver such as replacing an arthritic joint. Even in CRPS local anaesthetic blocks can temporarily abolish allodynia and spontaneous pain. [8]
The clinician should always have Epistemic Humility.[8] Our current understanding is evolving, and the tools for detecting both peripheral and central drivers have real limitations. Central conditions tend to be diagnosed by "committee criteria," i.e. a list of variably objective criteria with the final proviso being that other causes have been ruled out. I.e. it is when specific conditions are ruled out, not when the central condition has been ruled in. If the list of conditions to rule out is incomplete then the whole framework is wrong. Fibromyalgia was previously thought as being primarily a process driven by central sensitisation, but a large proportion of fibromyalgia patients actually have small fibre neuropathy on punch biopsy. I.e. for these patients they actually have a neurological disease, and many have been incorrectly told that their pain is central.[11]
Particular note must be made around chronic low back pain as it is the most common site of chronic musculoskeletal pain. Central sensitisation in chronic low back pain was found to be inconsistent and variable depending on the method used in a review of 17 studies.[12] The term "non-specific chronic low back pain" (NSCLBP) is often inappropriately used. Patients may be incorrectly diagnosed with NSCLBP (and resulting treatment being directed at postulated central processes) despite the fact that peripheral sources have not been ruled out using validated measures. For example they may have had an MRI, but not had medial branch blocks to assess for facet joint mediated pain, or sacroiliac joint blocks to assess for sacroiliac joint pain. In both these of these peripheral pain sources there are proven treatments available when the correct diagnosis is made (e.g. radiofrequency neurotomy for proven facet joint pain, and sacroiliac joint fusion for sacroiliac joint pain)
It is further important for the clinician to remember that many tools of diagnosis such as MRI do not have perfect inter-observer reliability. An example of this is how some radiologists in New Zealand do not report on lumbar spine lateral recess stenosis in radicular pain syndromes, and so the patient with lateral recess stenosis and radicular pain may incorrectly be labelled as having pain due to a central process.
Keeping the minefield above in mind, there are some features on assessment that can raise suspicion of a central physiologic process occurring. Neurological pain descriptors on history taking should be noted. On examination the clinician should look for decreased threshold, increased response, increased extent, and increased duration. Examination findings to look for are dynamic allodynia (e.g. stroking with a brush); secondary punctate hyperalgesia or pressure hyperalgesia; cold allodynia, regional and remote hyperalgesia; temporal summation and after sensations. "Non-dermatomal" sensory deficits may be noted, but it is important to keep in mind that the "dermatome" concept is only a model, and is not accurate in many people.
- Chronic musculoskeletal pain, and symptom/signs (assumed neurophysiology)
It is important to keep in mind that any combination may be present in a given individual.
| Pain Type | Distinguishing Features |
|---|---|
| Nociceptive |
|
| Peripheral neuropathic |
|
| Central (sensitisation) |
|
Differential Diagnosis
- Autoimmune: Polymyalgia Rheumatica, Sjogren's Disease, Rheumatoid Arthritis, Connective tissue disease (autoimmune), Inflammatory myositis, Sarcoidosis, Celiac disease, Inflammatory Bowel Disease, Sarcoidosis
- Genetic: Heritable Connective Tissue Disorders, Neuromuscular Diseases (e.g. Myotonic Dystrophy type 2, Pompe Disease)
- Idiopathic: Fibromyalgia, Degenerative myopathies, Small Fibre Neuropathy
- Primary Joint disease: Osteoarthritis
- Neoplastic disease: Metastases, Multiple myeloma, Leukaemia, Lymphoma
- Infections: bacterial endocarditis, hepatitis B and C
- Bone disease: Osteomalacia, Osteomyelitis
- Endocrine: Hypothyroidism and hyperthyroidism, Hyperparathyroidism, Acromegaly, Diabetes, Vitamin D deficiency, endocrine myopathies
- Neurological: Parkinson disease, Neuropathies, Motor neurone disease, Chronic Inflammatory Demyelinating Polyneuropathy, Multiple sclerosis, Myalgias in rare disease (e.g., Stiff Person Syndrome), Myalgias with lesions of the central and peripheral nervous system
- Medication: opioids, statins, aromatase inhibitors, carbimazole, ezitimibe, metoprolol, minoxidil, PPIs, salbutamol, amiodarone, colchicine, ACE inhibitors
Management
Treatment of Peripheral Nociceptive Driver
The presence of postulated sensitisation does not in itself preclude investigating and managing peripheral pain drivers.
An example is in post-traumatic chronic cervical pain (whiplash associated disorders). For example medial branch blocks result in a large and significant decrease in cold pain thresholds.[13] When performed in a rigorous manner, cervical radiofrequency neurotomy can be a very successful intervention. In a local study, a total of 104 patients selected on the basis of complete relief of pain following controlled, diagnostic, medial branch blocks, were treated with radiofrequency neurotomy. In the two practices 74% and 61% achieved at least 80% pain relief, with complete restoration of activities of daily living, no need for any further health care, and return to work.[14] Cervical radiofrequency neurotomy results in sustained improvements in pain, disability, local and widespread hyperalgesia, NFR threshold, brachial plexus provocation test responses, and range of motion.[15] Despite central processes possibly being prevalent, clearly the peripheral nociceptive driver is the underlying cause in many of these patients, with central processes being secondary and furthermore reversible once the peripheral cause is reversed.
There are multiple other examples in other pain conditions of reversal of sensitisation after effective surgical treatment of the peripheral driver, for example in knee osteoarthritis, hip osteoarthritis, and chronic low back pain.[16][17][18][19] Some patients may have even have widespread pain that is greatly improved after successful knee replacement surgery for knee osteoarthritis. Pain charts are indistinguishable from those that had failed surgery.[20]
Psychological Neuroscience Education
In one recent meta-analysis, pain neuroscience education when added to physiotherapy was found to have moderate evidence for a weak effect on pain in the short term. There was a 0.73 weighted mean difference reduction on a ten point scale but the confidence interval crosses 0. There was a small improvement in disability of 0.42 points. The analysis failed to show evidence of long-term improvement.[21]
Pharmacological
For fibromyalgia and neuropathic pain, cochrane found amitriptyline had an NNT of 4.6, duloxetine 8, pregabalin 9.8-11, and oxycodone had no evidence. Medication is often a hit or miss, bimodal response. It tends to not work for most people, but can work very well for the occasional person.
Psychological
CBT has a small benefit with pain reduction in fibromyalgia of 0.5 on 0-10 scale, and disability reduction of 0.7 on 0-10 scale.[22]
Prognosis
- Osteoarthritis: In patients with osteoarthritis of the knee or hip who undergo joint replacement, chronic widespread pain (CWP) correlates with poorer pain outcomes, and increased analgesic use.[1]
- Autoimmune and inflammatory rheumatological conditions: CWP is correlated with increased pain and worse outcomes when measured by self-report questionnaires.[1]
- Chronic low back pain: CWP is present in 25% of patients, and correlated with greater disability.[1]
- Chronic neck pain: CWP at one month post neck injury is correlated with poor outcomes at 6 months.[1]
- Carpal tunnel syndrome: worse outcomes with CWP[1]
- Shoulder pain: worse outcomes with CWP[1]
- Lateral elbow tendinopathy: Worse outcomes with CWP[1]
Neural injury and disease can induce permanent CNS changes as occurs in peripheral neuropathic pain. However, there is no good evidence that chronic unrelieved pain in the absence of neural pathology can "burn in" to the CNS. Also when there is an obvious peripheral source of pain such as hip osteoarthritis, the pain is abolished with joint arthroplasty (except in cases of chronic widespread pain as above). This is further demonstrated by the success of radiofrequency neurotomy in persistent facet mediated pain. However what can occur in unrelieved pain is psychosocial deterioration and financial distress.[23]
Resources
See Also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Goldenberg, D et al. Overview of chronic widespread (centralized) pain in the rheumatic diseases. In: UpToDate, Post, TW (Ed), UpToDate, Waltham, MA, Jan 23 2020.
- ↑ Dominick et al.. Patterns of chronic pain in the New Zealand population. The New Zealand medical journal 2011. 124:63-76. PMID: 21946879.
- ↑ International Association for the Study of Pain (2020) IASP’s New Definition of Pain. Available at: https://www.iasp-pain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=10475 (accessed 27 July 2020).
- ↑ Woolf. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011. 152:S2-15. PMID: 20961685. DOI. Full Text.
- ↑ Woolf et al.. Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. Journal de physiologie 1988. 83:255-66. PMID: 3272296.
- ↑ Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005 Mar;114(1-2):295-302. doi: 10.1016/j.pain.2004.12.032. PMID: 15733656.
- ↑ Oaklander et al.. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013. 154:2310-6. PMID: 23748113. DOI. Full Text.
- ↑ 8.0 8.1 8.2 8.3 van Griensven et al.. Central Sensitization in Musculoskeletal Pain: Lost in Translation?. The Journal of orthopaedic and sports physical therapy 2020. 50:592-596. PMID: 33131390. DOI.
- ↑ Coronado & George. The Central Sensitization Inventory and Pain Sensitivity Questionnaire: An exploration of construct validity and associations with widespread pain sensitivity among individuals with shoulder pain. Musculoskeletal science & practice 2018. 36:61-67. PMID: 29751194. DOI. Full Text.
- ↑ Adams, Greig R; Gandhi, Wiebke; Harrison, Richard; van Reekum, Carien M; Wood-Anderson, Desmond; Gilron, Ian; Salomons, Tim V. (2022-12-06). "Do "Central Sensitisation" Questionnaires Reflect Measures of Nociceptive Sensitisation or Psychological Constructs? a Systematic Review and Meta-Analyses:". Pain (in English). Publish Ahead of Print. doi:10.1097/j.pain.0000000000002830. ISSN 0304-3959.
- ↑ Martínez-Lavín. Fibromyalgia and small fiber neuropathy: the plot thickens!. Clinical rheumatology 2018. 37:3167-3171. PMID: 30238382. DOI.
- ↑ Roussel et al.. Central sensitization and altered central pain processing in chronic low back pain: fact or myth?. The Clinical journal of pain 2013. 29:625-38. PMID: 23739534. DOI.
- ↑ Schneider et al.. Minimizing the source of nociception and its concurrent effect on sensory hypersensitivity: an exploratory study in chronic whiplash patients. BMC musculoskeletal disorders 2010. 11:29. PMID: 20144214. DOI. Full Text.
- ↑ MacVicar et al.. Cervical medial branch radiofrequency neurotomy in New Zealand. Pain medicine (Malden, Mass.) 2012. 13:647-54. PMID: 22458772. DOI.
- ↑ Smith et al.. Cervical radiofrequency neurotomy reduces central hyperexcitability and improves neck movement in individuals with chronic whiplash. Pain medicine (Malden, Mass.) 2014. 15:128-41. PMID: 24138594. DOI.
- ↑ Kosek & Ordeberg. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. European journal of pain (London, England) 2000. 4:229-38. PMID: 10985866. DOI.
- ↑ Graven-Nielsen et al.. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis and rheumatism 2012. 64:2907-16. PMID: 22421811. DOI.
- ↑ Aranda-Villalobos et al.. Normalization of widespread pressure pain hypersensitivity after total hip replacement in patients with hip osteoarthritis is associated with clinical and functional improvements. Arthritis and rheumatism 2013. 65:1262-70. PMID: 23400951. DOI.
- ↑ Seminowicz et al.. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. The Journal of neuroscience : the official journal of the Society for Neuroscience 2011. 31:7540-50. PMID: 21593339. DOI. Full Text.
- ↑ Skou et al.. Widespread sensitization in patients with chronic pain after revision total knee arthroplasty. Pain 2013. 154:1588-1594. PMID: 23707268. DOI.
- ↑ Wood & Hendrick. A systematic review and meta-analysis of pain neuroscience education for chronic low back pain: Short-and long-term outcomes of pain and disability. European journal of pain (London, England) 2019. 23:234-249. PMID: 30178503. DOI.
- ↑ Bernardy et al.. Cognitive behavioural therapies for fibromyalgia. The Cochrane database of systematic reviews 2013. CD009796. PMID: 24018611. DOI. Full Text.
- ↑ Marshall Devor. Peripheral Neuropathic Pain. Encyclopedia of Pain 2012.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,