Numbness
Anatomy
- Main article: Spinal Cord Anatomy
Part or all of this article or section is derived from Numbness by ddxof, used under CC-BY-SA
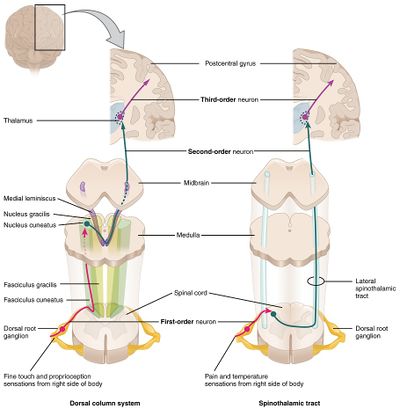
Sensory information is gathered from specialized receptors in skin and soft-tissues which detect a variety of stimuli including temperature, pressure, vibration, and pain. This is subsequently transmitted through peripheral nerves which in certain regions coalesce into larger bundles before entering the spinal cord through the dorsal nerve root. Upon entering the spinal cord, sensory information is divided into two tracts:
- Spinothalamic: Small, poorly-myelinated fibers carrying pain, temperature and touch stimuli synapse with second-order neurons over several levels in the ipsilateral dorsal horn crossing to the contralateral side in the anterior commissure anterior to the central canal. Touch information ascends in the anterior spinothalamic tract while pain and temperature information ascends in the lateral spinothalamic tract.
- Dorsal column: Large, myelinated fibers carrying vibration and proprioception information ascend in the ipsilateral posteromedial spinal cord. Fibers from the thoracic and lumbar region occupy the more medial (gracile) column, while fibers from the cervical region occupy the more lateral (cuneate) column. These fibers synapse in their respective nuclei in the medulla before crossing to the contralateral medial lemniscus.
Both tracts proceed to the ventral posterolateral (VPL) nucleus of the thalamus, through the internal capsule before terminating in the somatotopically-arranged primary somatosensory cortex in the parietal lobe.
Understanding the neuroanatomy supports a systematic approach to the evaluation of sensory disturbances. It is important to note that the transmission of light touch sensation involves both pathways and offers less localizing value when compared to specific assessment of proprioception or pain detection. Sensory disturbances are often accompanied by motor abnormalities which can further aid with localization. Other key distinguishing features include acuity of onset wherein abrupt presentations may suggest ischemia or infarction, compared to more indolent processes with broader differentials (including compressive mass lesions, demyelination, or autoimmune disease).
Aetiology
- Peripheral nerve (e.g. carpal tunnel syndrome)
- Nerve root (e.g. C6 radiculopathy)
- Multiple nerves
- Polyneuropathy
- mononeuritis multiplex
- Spinal cord
- Complete spinal cord injury
- Incomplete spinal cord lesions (anterior cord syndrome, brown-Sequard, syringomyelia)
- Lateral medullary infarction
- Thalamic disease
- Cerebral hemispheric disease
Cognitive Approach
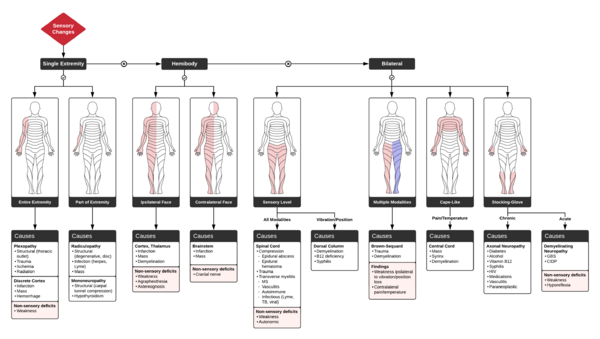
When assessing sensory syndromes, one should ask the following questions
A. DOES THE SENSORY LOSS INVOLVE BOTH SIDES OF THE BODY?
- Bilateral involvement: suggests polyneuropathy or spinal cord disease
- One sided involvement: Indicates contralateral brainstem, thalamus, or cerebral cortex
- Mixed: Pain and temperature loss on one side and tactile sensory loss on the contralateral side suggests hemisection of the spinal cord (on tactile loss side).
B. IS THERE A SENSORY LEVEL?
- Sensory level = distinct border on trunk below which sensory testing is abnormal
- Suggests spinal cord disease or lateral medullary infarction
C. IS THERE SENSORY DISSOCIATION?
- Loss of pain and temperature sensation PLUS Preservation of touch and vibratory sense
- Suggests incomplete spinal cord syndrome (syringomyelia, spinal stroke, Brown-Sequard)
D. IS THERE SENSORY LOSS ON THE FACE?
- Suggests lesion above the spinal cord in the brainstem, thalamus, or cerebral hemispheres.
- Brainstem causes loss opposite to lesion
- Thalamus and cerebral hemisphere causes loss ipsilateral to lesion
E. ARE THERE ASSOCIATED NEUROLOGIC SIGNS?
- Especially weakness, most sensory syndromes cause significant weakness (except lateral medullary syndrome)
- Horner syndrome suggests ipsilateral brainstem or cervical spinal cord