Opioids: Difference between revisions
No edit summary |
mNo edit summary |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Curriculum}}{{partial}} | ||
==Classification== | Opioids are an analgesic, but have fell out of favour in the treatment of chronic pain in New Zealand. They currently only have a role in acute pain and chronic cancer related pain. Before the onset of tolerance, opioids exert their analgesic effects through peripheral, spinal, and supraspinal sites. | ||
== Definitions == | |||
''Opiate'': any compound that is structurally related to products found in opium. Opiates include the natural plant alkaloids such as morphine and codeine. | |||
''Opioid'': any agent regardless of its structure that has the functional and pharmacological properties of an opiate. | |||
''Endogenous opioids'': Naturally occurring ligands that act on opioid receptors. | |||
''Endorphin'': Often used synonymously with endogenous opioid but also refers to the specific opioid β-Endorphin | |||
''Narcotic'': Originally referred to any drug that induced narcosis or sleep, now associated with opioids often used in a legal context to refer to a variety of substances that have an abuse or addictive potential. | |||
== Classification == | |||
[[File:Opioid-chemical-classification.png|thumb|Chemical classification of opioids. Copyrighted<ref>Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008 Mar;11(2 Suppl):S133-53. PMID: 18443637.</ref>]] | |||
'''Classification by chemistry''' | |||
There are four base molecules that give rise to four families of opioids | |||
* Phenathrenes - prototypical opioids such as morphine, codeine, oxycodone | |||
* Diphenylheptanes- methadone and propoxyphene | |||
* Phenylpiperidines - pethidine, fentanyl, and other fentayl related compounds | |||
* Benzomorphans - pentazocine only | |||
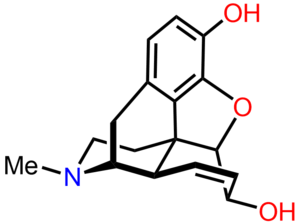
[[File:Morphine chemical structure in 3D.png|left|thumb|Morphine structure in three dimensions]] | |||
Morphine is the archetypal opioid and is the reference standard against for other opioids. It has a benzene ring with a phenolic hydroxyl group at position 3, as well as an alcohol hydroxyl groups at position 6 and at the nitrogen atom. Both of the position 3 and 6 hydroxyl groups can be converted to ethers or esters forming other compounds. The nitrogen group is in a tertiary form and this is important to its analgesic properties. | |||
Tramadol doesn't fit into the above classes. It is an atypical opioid with partial mu agonist activity and central CABA, catecholamine, and serotonergic activities. | |||
'''Classification by receptor affinity''' | '''Classification by receptor affinity''' | ||
| Line 19: | Line 47: | ||
*Synthetic compounds - Pethidine, Fentanyl, Methadone, Alfentanil, Remifentanil, Tapentadol | *Synthetic compounds - Pethidine, Fentanyl, Methadone, Alfentanil, Remifentanil, Tapentadol | ||
== | ==Mechanism of Action== | ||
'''Endogenous Opioids''' | |||
There are four known distinct families of endogenous opioid peptides, the most studied of which are the '''enkephalins''', '''endorphins''', and '''dynorphins'''. They function as neurotransmitters in the pleasure-reward and pain pathways, and may have a role with hormone secretion, thermoregulation, and cardiovascular control. | |||
They all derive from their own large precursor protein. Enkephalins derive from Proenkephalin, endorphins derive from Pro-opiomelanocortin (POMC), and dynorphins derive from Prodynorphin. A fourth more recently discovered family are the '''endomorphins''', but the precursor is unknown. | |||
'''Opioid Receptors''' | |||
There are three main types of opioid receptors: μ (MOP), δ (DOP), and κ (KOP). They belong to the rhodopsin family of GPCRs. The natural ligands interact with each receptor but don't do so exclusively. These are the endomorphins and β-Endorphin (μ), enkephalins (δ), and dynorphins (κ). | |||
All receptors have analgesic effects, but most opioids with clinical relevance have primary agonist activity on μ (mu) receptors, and so are termed μ agonists. With increasing dose, analgesia theoretically occurs in a log linear fashion. While opioid agonists/antagonists and partial agonists have a ceiling effect. | |||
Opioid receptors are found within the CNS as well as in peripheral tissues. | |||
{| class="wikitable" | {| class="wikitable" | ||
|+ | |+ | ||
Effects of Opioid Receptors<ref>Modified from Millers Anaesthesia</ref><ref>Brunton et al. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 2011</ref> | |||
| | | | ||
|style="width: 28%"|'''Mu''' | | style="width: 28%" |'''Mu (µ)''' | ||
|style="width: 28%"|'''Delta''' | | style="width: 28%" |'''Delta (δ)''' | ||
|style="width: 28%"|'''Kappa''' | | style="width: 28%" |'''Kappa (κ)''' | ||
|- | |- | ||
| | | | ||
|Mu1 - | |Mu1 - Supraspinal analgesia | ||
Mu2 - Sedation, vomiting, respiratory depression, pruritis, euphoria, anorexia, urinary retention, physical dependence. | Mu2 - Sedation, vomiting, respiratory depression, pruritis, euphoria, stress coping, anorexia, urinary retention, physical dependence. | ||
| | |Spinal analgesia. Anxiolytic, positive affect | ||
| | |Spinal analgesia, sedation, dyspnoea, miosis, respiratory depression, dysphoria, negative affect, dependence | ||
|- | |- | ||
! colspan="4" |'''Endogenous Peptides''' | ! colspan="4" |'''Endogenous Peptides''' | ||
|- | |- | ||
|Enkephalins | |Enkephalins | ||
| | | ++ | ||
| | | +++ | ||
| | | | ||
|- | |- | ||
|β-Endorphin | |β-Endorphin | ||
| | | +++ | ||
| | | +++ | ||
| | | | ||
|- | |- | ||
|Dynorphin A | |Dynorphin A | ||
| | | ++ | ||
| | |||
| +++ | |||
|- | |||
|Dynorphin B | |||
| + | |||
| + | |||
| +++ | |||
|- | |||
|Endomorphin-1 | |||
| +++ | |||
| | |||
| | | | ||
|- | |- | ||
! colspan="4" |'''Agonists''' | ! colspan="4" |'''Agonists''' | ||
|- | |- | ||
|Morphine | |Morphine | ||
| | | +++ | ||
| | | | ||
| + | |||
|- | |- | ||
|Fentanyl | |Fentanyl | ||
| | | +++ | ||
| | | | ||
| | | | ||
|- | |- | ||
|Methadone | |Methadone | ||
| | | +++ | ||
| | | | ||
| | | | ||
| Line 81: | Line 122: | ||
|- | |- | ||
|Naloxone | |Naloxone | ||
| | |−−− | ||
| | |− | ||
| | |−− | ||
|- | |- | ||
|Naltrexone | |Naltrexone | ||
| | |−−− | ||
| | |− | ||
| | |−−− | ||
|} | |} | ||
'''Supraspinal Effects''' | |||
Opioid receptors are highly localised in subcortical regions (thalamus, periaqueductal gray, rostral ventromedial medulla, and locus ceruleus). Descending pain-modulating pathways originate from these areas which are activated with opioids. | |||
Opioids block the release of GABA from presynaptic inhibitory inter-neurons in these descending pain pathways. This leads to the activation of monoamine-containing descending neurons that supress nociceptive transmission in the dorsal horn. A reduction in GABA is also accompanied by release of dopamine leading to opioid addiction in some individuals. | |||
Opioids can also antagonise NDMA receptors, thereby activating the descending serotonin and noradrenaline pain pathways. NMDA receptors are thought to be involved in neuropathic pain and the development of tolerance.<ref name=":0">Trang, Tuan et al. “Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics.” ''The Journal of neuroscience : the official journal of the Society for Neuroscience'' vol. 35,41 (2015): 13879-88. doi:10.1523/JNEUROSCI.2711-15.2015</ref> | |||
'''Spinal Effects''' | |||
They are also found on presynaptic afferent fibres, interneurons, and postsynaptic projection neurons. In the spinal cord they are found in the dorsal horn, primarily in laminae I and II. | |||
Presynaptically, opioids bind to presynaptic opioid receptors located on C and Aδ neuronal membranes, reducing cAMP signalling and suppressing the activity of voltage-gated calcium channels. They thereby prevent the release of endogenous pain neurotransmitters such as glutamate, substance P, and calcitonin gene-related peptide. | |||
Postsynaptically, opioids activate inward potassium channels to cause hyperpolarisation of ascending projection neurons.<ref name=":0" /> | |||
== Specific Opioids == | == Specific Opioids == | ||
'''Morphine''' | '''Morphine''' | ||
| Line 370: | Line 395: | ||
==Adverse Effects== | ==Adverse Effects== | ||
{{See also|Opioid Deprescribing}} | {{See also|Opioid Deprescribing}} | ||
The evidence | Many of the long term effects come about from long term use. The evidence is clear that long term opioid use is not indicated in chronic non-malignant pain. | ||
Long term use of opioids is associated with tolerance and opioid induced hyperalgesia. There is activation of pain facilitatory systems from the medulla that opposes the analgesic action, a "physiological antagonist of analgesia". With the activation of descending pain facilitatory pathways there can also be the induction of a paradoxical hyperalgesic state. This is termed opioid induced hyperalgesia. If unrecognised it can lead to ever increasing doses of opioids. | |||
There are numerous other adverse effects such as neurohumoral effects which include hormonal dysregulation, immunosuppression, constipation, urinary retention, cardiovascular depression, motor system effects, respiratory depression, increased risk of falls, and impairment of sleep quality. | |||
==References== | ==References== | ||
[[Category:Pharmacology]] | [[Category:Pharmacology]] | ||
Latest revision as of 20:23, 18 March 2022
Opioids are an analgesic, but have fell out of favour in the treatment of chronic pain in New Zealand. They currently only have a role in acute pain and chronic cancer related pain. Before the onset of tolerance, opioids exert their analgesic effects through peripheral, spinal, and supraspinal sites.
Definitions
Opiate: any compound that is structurally related to products found in opium. Opiates include the natural plant alkaloids such as morphine and codeine.
Opioid: any agent regardless of its structure that has the functional and pharmacological properties of an opiate.
Endogenous opioids: Naturally occurring ligands that act on opioid receptors.
Endorphin: Often used synonymously with endogenous opioid but also refers to the specific opioid β-Endorphin
Narcotic: Originally referred to any drug that induced narcosis or sleep, now associated with opioids often used in a legal context to refer to a variety of substances that have an abuse or addictive potential.
Classification
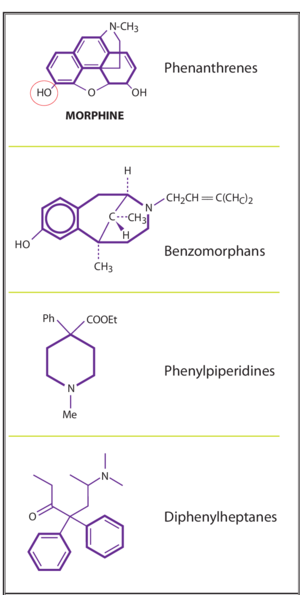
Classification by chemistry
There are four base molecules that give rise to four families of opioids
- Phenathrenes - prototypical opioids such as morphine, codeine, oxycodone
- Diphenylheptanes- methadone and propoxyphene
- Phenylpiperidines - pethidine, fentanyl, and other fentayl related compounds
- Benzomorphans - pentazocine only
Morphine is the archetypal opioid and is the reference standard against for other opioids. It has a benzene ring with a phenolic hydroxyl group at position 3, as well as an alcohol hydroxyl groups at position 6 and at the nitrogen atom. Both of the position 3 and 6 hydroxyl groups can be converted to ethers or esters forming other compounds. The nitrogen group is in a tertiary form and this is important to its analgesic properties.
Tramadol doesn't fit into the above classes. It is an atypical opioid with partial mu agonist activity and central CABA, catecholamine, and serotonergic activities.
Classification by receptor affinity
- Opioid Agonists - Morphine, Codeine, Meperidine, Fentanyl, Sufentanil, Remifentanil, Methadone, Tramadol
- Opioid Agonist/Antagonists & Partial Agonists - Pentazocine, Nalbuphine, Butorphanol, Buprenorphine
- Opioid Antagonists - Nalorphine, Naloxone, Naltrexone, Naltrindole, Nalmefene
Classification by Exogenous Opioids Strength
According to the strength or potency based on the plasma concentrations at which they exert their effects. C50 or the plasma concentration causing a 50% effect.
- Strong Opioids include fentanyl, sufentanil, and remifentanil
- Weak opioids include codeine and tramadol
- Intermediate group includes morphine, methadone, oxycodone, and buprenorphine
Classification by synthetic process
- Naturally occurring compounds - Morphine, Codeine, Thebaine, Papaverine
- Semi-synthetic compounds - Diamorphine (heroin), Dihydromorphone, Buprenorphine, Oxycodone
- Synthetic compounds - Pethidine, Fentanyl, Methadone, Alfentanil, Remifentanil, Tapentadol
Mechanism of Action
Endogenous Opioids
There are four known distinct families of endogenous opioid peptides, the most studied of which are the enkephalins, endorphins, and dynorphins. They function as neurotransmitters in the pleasure-reward and pain pathways, and may have a role with hormone secretion, thermoregulation, and cardiovascular control.
They all derive from their own large precursor protein. Enkephalins derive from Proenkephalin, endorphins derive from Pro-opiomelanocortin (POMC), and dynorphins derive from Prodynorphin. A fourth more recently discovered family are the endomorphins, but the precursor is unknown.
Opioid Receptors
There are three main types of opioid receptors: μ (MOP), δ (DOP), and κ (KOP). They belong to the rhodopsin family of GPCRs. The natural ligands interact with each receptor but don't do so exclusively. These are the endomorphins and β-Endorphin (μ), enkephalins (δ), and dynorphins (κ).
All receptors have analgesic effects, but most opioids with clinical relevance have primary agonist activity on μ (mu) receptors, and so are termed μ agonists. With increasing dose, analgesia theoretically occurs in a log linear fashion. While opioid agonists/antagonists and partial agonists have a ceiling effect.
Opioid receptors are found within the CNS as well as in peripheral tissues.
| Mu (µ) | Delta (δ) | Kappa (κ) | |
| Mu1 - Supraspinal analgesia
Mu2 - Sedation, vomiting, respiratory depression, pruritis, euphoria, stress coping, anorexia, urinary retention, physical dependence. |
Spinal analgesia. Anxiolytic, positive affect | Spinal analgesia, sedation, dyspnoea, miosis, respiratory depression, dysphoria, negative affect, dependence | |
| Endogenous Peptides | |||
|---|---|---|---|
| Enkephalins | ++ | +++ | |
| β-Endorphin | +++ | +++ | |
| Dynorphin A | ++ | +++ | |
| Dynorphin B | + | + | +++ |
| Endomorphin-1 | +++ | ||
| Agonists | |||
| Morphine | +++ | + | |
| Fentanyl | +++ | ||
| Methadone | +++ | ||
| Antagonists | |||
| Naloxone | −−− | − | −− |
| Naltrexone | −−− | − | −−− |
Supraspinal Effects
Opioid receptors are highly localised in subcortical regions (thalamus, periaqueductal gray, rostral ventromedial medulla, and locus ceruleus). Descending pain-modulating pathways originate from these areas which are activated with opioids.
Opioids block the release of GABA from presynaptic inhibitory inter-neurons in these descending pain pathways. This leads to the activation of monoamine-containing descending neurons that supress nociceptive transmission in the dorsal horn. A reduction in GABA is also accompanied by release of dopamine leading to opioid addiction in some individuals.
Opioids can also antagonise NDMA receptors, thereby activating the descending serotonin and noradrenaline pain pathways. NMDA receptors are thought to be involved in neuropathic pain and the development of tolerance.[4]
Spinal Effects
They are also found on presynaptic afferent fibres, interneurons, and postsynaptic projection neurons. In the spinal cord they are found in the dorsal horn, primarily in laminae I and II.
Presynaptically, opioids bind to presynaptic opioid receptors located on C and Aδ neuronal membranes, reducing cAMP signalling and suppressing the activity of voltage-gated calcium channels. They thereby prevent the release of endogenous pain neurotransmitters such as glutamate, substance P, and calcitonin gene-related peptide.
Postsynaptically, opioids activate inward potassium channels to cause hyperpolarisation of ascending projection neurons.[4]
Specific Opioids
Morphine
- Low lipid solubility
- Slow onset action (slow BBB penetration)
- High 1st pass metabolism (LIVER)
- Metabolism CYP450 / glucuronidation
- 40-60% reach systemic circulation
- Renal excretion
Methadone
- Longer duration action
- Limited first pass metabolism
- High bioavailability
- ANTAGONIST NMDA receptor – Neuropathic pain
- Inhibits re-uptake NA/Serotonin
- Metabolised in Liver / excretes feaces (mostly)
Tramadol
- Phenylpiperidine analogue (Codeine)
- Modulation Serotonin and NA reuptake
- Less Respiratory depression
- Less GIT S/E (for comparable analgesic effect)
Tapentadol
- MOP receptor agonist
- NA reuptake inhibition
- Comparable opioid potency to Oxycodone
Ramifentanyl
- High Lipid solubility
- Rapid extra-hepatic metabolism
Codeine
- “Pro-drug”
- Converted to morphine for effect
- 5-10% population unable to convert / ineffective
Pethidine (Meperidine)
- Relative weak opioid (10% morphine)
- ++ anticholinergic effects
- Neurotoxic S/E (Particularly in renal disease)
- Delerium
- Excitation
- Seizures
Opioid Potency Comparison
| Opioid Agonists | Potency | Half-life |
|---|---|---|
| Morphine | 1 | 2 - 3.5 |
| Hydromorphone | 5 - 8 | 2 - 3 |
| Oxycodone | 1.5 | 2 - 3 |
| Methadone | 1 | 24 |
| Fentanyl | 100 | 3 - 4 |
| Codeine | 0.13 | 3 |
| Tramadol | 0.2 | |
| Sufentanil | 1000 | |
| Alfentanil | 20 | |
| Remifentanil | 100 |
| Converting | Ratio | Example | ||
|---|---|---|---|---|
| from | to | |||
| Codeine oral | morphine oral | 10:1 | Codeine 60mg oral | -> morphine 6mg oral |
| Tramadol oral | morphine oral | 10:1 | Tramadol 100mg oral | -> morphine 10mg oral |
| Dihydrocodeine oral | Morphine oral | 10:1 | Dihydrocodeine 100mg oral | -> Morphine 10mg oral |
| Oxycodone oral | Morphine oral | 1:1.5 | Oxycodone 10mg oral | -> Morphine 15mg oral |
| Morphine oral | Oxycodone oral | 2:1 | Morphine 20mg oral | -> Oxycodone 10mg oral |
| Morphine oral | Morphine subcut | 2 to 3:1 | Morphine 30mg oral | -> Morphine 10-15mg subcut |
| Oxycodone oral | Oxycodone subcut | 2:1 | Oxycodone 20mg oral | -> Oxycodone 10mg subcut |
| Oxycodone subcut | Morphine subcut | 1:1 | Oxycodone 10mg subcut | -> Morphine 10mg subcut |
| Morphine oral | Fentanyl subcut | 100:1 | Morphine 30mg oral | -> Fentanyl 0.3mg subcut |
Calculation of oral Morphine Equivalent Daily Dose (oMEDD)
oMEDD (mg) = Current Opioid Dose x Conversion factor
Renal Failure
| % Normal Dose | Dose (mg) | Dose Interval (hourly) | |
|---|---|---|---|
| Mild renal impairment (GFR 20-50) | 75% | 2.5-5 | 6 |
| Moderate renal impairment (10-20) | 50% | 2.5-5 | 6-8 |
| Severe renal impairment (<10) | Use small doses | 1.25-2.5 | 8-12 |
| % Normal Dose | Dose (mg) | Dose Interval (hourly) | |
|---|---|---|---|
| Mild renal impairment (GFR 20-50) | 100% | 50-100 | 6 |
| Moderate renal impairment (10-20) | 50% | 50-100 | 6-8 (modify as needed) |
| Severe renal impairment (<10) | 50% | 50 | 6-8 (modify as needed) |
| % Normal Dose | Dose (mg) | Dose Interval (hourly) | |
|---|---|---|---|
| Mild renal impairment (GFR 20-50) | 50% | 2.5-5 | 6 |
| Moderate renal impairment (10-20) | 25-50% | 2.5-5 | 6-8 (modify as needed) |
| Mild renal impairment (<10) | 25-50% | 2.5-5 | 8-12 (modify as needed |
Fentanyl in Renal Failure
Fentanyl is metabolised in the liver to inactive metabolites. It is a useful Strong opioid in renal failure with stable pain. Fentanyl is available in patches.
Liver Failure
There is variable time of onset and analgesic efficacy. They are reasonably well tolerated in adjusted dosing. Ramifentanyl has no hepatic metabolism. Fentanyl patches are a good choice.
Variation in Sensitivity
Polymorphism in human OPRM1 gene which encodes the mu opoid receptor might contribute to variation in sensitivity.
Adverse Effects
- See also: Opioid Deprescribing
Many of the long term effects come about from long term use. The evidence is clear that long term opioid use is not indicated in chronic non-malignant pain.
Long term use of opioids is associated with tolerance and opioid induced hyperalgesia. There is activation of pain facilitatory systems from the medulla that opposes the analgesic action, a "physiological antagonist of analgesia". With the activation of descending pain facilitatory pathways there can also be the induction of a paradoxical hyperalgesic state. This is termed opioid induced hyperalgesia. If unrecognised it can lead to ever increasing doses of opioids.
There are numerous other adverse effects such as neurohumoral effects which include hormonal dysregulation, immunosuppression, constipation, urinary retention, cardiovascular depression, motor system effects, respiratory depression, increased risk of falls, and impairment of sleep quality.
References
- ↑ Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008 Mar;11(2 Suppl):S133-53. PMID: 18443637.
- ↑ Modified from Millers Anaesthesia
- ↑ Brunton et al. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 2011
- ↑ 4.0 4.1 Trang, Tuan et al. “Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics.” The Journal of neuroscience : the official journal of the Society for Neuroscience vol. 35,41 (2015): 13879-88. doi:10.1523/JNEUROSCI.2711-15.2015