Osteonecrosis: Difference between revisions
No edit summary |
mNo edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{partial}} | |||
Osteonecrosis refers to cell death within bones that occurs from a lack of circulation rather than from disease. The structural components are unaffected. The cells include osteocytes, and usually the marrow contents. | Osteonecrosis refers to cell death within bones that occurs from a lack of circulation rather than from disease. The structural components are unaffected. The cells include osteocytes, and usually the marrow contents. | ||
| Line 167: | Line 167: | ||
* Orthopaedic Basic Sciences 2nd Edition. Chapter 14 Bone Injury Regeneration and Repair | * Orthopaedic Basic Sciences 2nd Edition. Chapter 14 Bone Injury Regeneration and Repair | ||
[[Category:Bone and Cartilage Disorders]] | |||
Latest revision as of 11:44, 2 August 2021
Osteonecrosis refers to cell death within bones that occurs from a lack of circulation rather than from disease. The structural components are unaffected. The cells include osteocytes, and usually the marrow contents.
Related Terms
Osteonecrosis is preferred over other terms avascular necrosis, ischaemic necrosis, and aseptic necrosis because it is more accurate histolo-pathologically and doesn't imply any specific aetiology. Historically, ischaemic and avascular necrosis was reserved for subchondral (epiphyseal) osteonecrosis, while bone infarct was referred to as medullary (metaphyseal) osteonecrosis. Osteonecrosis is a more general and inclusive term.
Pathogenesis
Osteonecrotic bone is not avascular as the vessels are still present. However in all mechanisms of osteonecrosis there is compromise of the circulation. There are four possible mechanisms of compromise
- Mechanical disruption of the vessels: From fracture or dislocation, or from atraumatic events such as stress or fatigue fractures
- Occlusion of the arterial vessels: From thrombosis, embolism, circulating fat, nitrogen bubbles, or abnormally shaped cells (sickle cell crises).
- Injury to or pressure on the arterial wall: From damage within the wall due to vasculitis or radiation injury; from damage within the vessel from release of materials causing angiospasm; or from external pressure or chemical reaction on the wall in extravasated blood, fat, or cellular elements in the marrow cavity.
- Occlusion to the venous outflow vessels: From venous pressure exceeded arterial pressure, may be mitigated by collateral circulation if sufficient.
Pathophysiology
Cellular Bone Injury
Regardless of the inciting disease or disorder, the processes of osteonecrosis are similar.
- Within the first few days to weeks after vascular compromise there are no histologic changes.
- In the second week there is the beginning of necrosis of cells within the marrow.
- The osteocytes shrink producing empty lacunae.
- Cell death within fatty marrow results in the release of lysosomes and the tissue becomes acidic.
- There is saponification from calcium and free fatty acids released from dead lipocytes.
- In normal fatty marrow there is little water. After necrosis there is increased water content which can be seen on MRI.
Repair of Osteonecrotic Bone
Repair is only initiated if the surrounding viable cellular tissue receives a repair signal.
In some areas with a small area of cell death there is no signal for repair generated and the bone infarct is permanent. If this area is small enough and it doesn't compromise the structural integrity of the bone then it may be asymptomatic and non-progressive. This commonly occurs with necrosis exclusively of the medullary bone without subchondral plate involvement. On imaging, calcification of saponified fats may be seen permanently on radiographs, fluid signal changes may be seen on MRI, and peripheral reactive changes may be seen on bone scan.
With osteonecrosis of cancellous bone there is a reparative response. Initially there is a reactive hyperaemia and vascular fibrosis repair process in the adjacent bony tissues. Creeping substitution then occurs which is identical to the process that occurs with the incorporation of bone grafts. Creeping substitution is the slow near-complete resorption of dead bone (or graft) with the simultaneous deposition of new viable bone. Within a few weeks there is revascularisation of the necrotic cancellous bone from the adjacent fibrous tissue. Vessels grow into the medullary canal and revascularise the cancellous bone and the haversian canals.
Within necrotic cancellous bone we see the production of osteoid on the scaffolding of the necrotic trabeculae. The new viable bone thickens the trabeculae, and there is an early increase in bone density, but not necessarily the same strength as normal bone with similar bone density. This increased density is seen on plain films by 6 to 12 months.
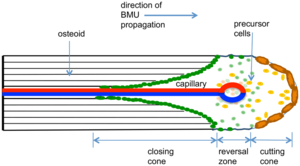
In the cortical bone the histologic appearance is of cutting cones, similar to what is seen at the metaphyseal end of growth plates. The bone in the haversian canals are resorbed, and new bone is produced. Mononuclear cells fuse to form osteoclasts, and resorb the necrotic haversian bone following vascular invasion. There is resorption of the majority of haversian bone but almost none of the interlamellar bone. In this stage the bone initially becomes osteoporotic, and fractures can occur at 18 to 24 months after the onset of necrosis. Osteoblasts subsequently replace the haversian system and the bone regains its original density after it has been repaired which takes at least 2 years.
In both cancellous and cortical bone mesenchymal cells differentiate into osteoblasts and osteoclasts.
Structural Sequelae
There is initially no alteration in the mechanical properties of necrotic bone because osteonecrosis only directly affects the cells of the bone.
If osteonecrosis involves subchondral bone, there is eventual fracture and collapse of the bone which leads to articular surface irregularities and ultimately degenerative osteoarthritis. This process is a secondary mechanical phenomenon. It occurs because with the absence of cells, the bone can't respond to the the loading of normal daily activities. The fracture and collapse are a result of multiple fatigue fractures due to repetitive loading of the bone, and these fractures can't be repaired.
Fatigue fractures occur in the following areas
- Where the remaining avascular subchondral trabeculae are oriented perpendicularly to the joint surface without the ability to repair small microfractures
- Where the resorption of the subchondral bone at the periphery, where there is vascular invasion of the necrotic bone, resulting in osteoporotic bone
- At the junction of increased bone density at the front of revascularisation results in a stress riser between it and adjacent avascular bone
Microfractures are able to elicit a pain response prior to complete fracture and collapse. If functional activities are reduced, then viable bone within or surrounding the osteonecrotic bone undergoes a reduction in bone mass. On plain films necrotic bone can be seen as a relative increased density area of unresorbed necrotic bone with adjacent viable osteoporotic bone.
With the onset of collapse of necrotic bone, the crescent sign appears on plain films reflecting the loss of subchondral support.
Aetiology
The cause of osteonecrosis in most cases is not completely understood. The most common causes of nontraumatic osteonecrosis are idiopathic, and in association with ethanol and corticosteroids. The mechanisms is unknown.
- Fracture or dislocation: The most common traumatic injuries leading to osteonecrosis are fractured femoral neck, dislocation of the femoral head, displaced fracture of the scaphoid, displaced fracture of the talar neck, and a 4-part fracture of the humeral head. There are clinically significant secondary complications in the form of collapse of the subchondral bone and adjacent articular surface.
- Infection: In osteomyelitis and pyarthrosis there is a combination of increased intramedullary pressure and arterial occlusion.
- Gaucher's disease: The marrow cavity is packed with Gaucher's cells which are macrophages filled with cerebroside. This leads to direct occlusion of intraosseous arteries.
- Sickle cell disease: The marrow cavity is packed with sickle shaped red blood cells. This also leads to direct occlusion of intraosseous arteries.
- Decompression sickness (caisson disease): There is likely vascular occlusion by nitrogen bubbles coming out of solution with a rapid drop in barometric pressure
- Radiation: This occurs from radiation damage to the capillaries
- Ethanol
- Corticosteroid administration
- Hyperlipidaemia
- Pancreatitis
- Idiopathic osteonecrosis: One theory is intraosseous hypertension with excessive pressure within the medullary space resulting in occlusion of intraosseous vessels.
Eponymous Names for Specific Sites
- Ahlback disease: medial femoral condyle, i.e. SONK
- Brailsford disease: head of the radius
- Buchman disease: iliac crest
- Burns disease: distal ulna
- Caffey disease: entire carpus or intercondylar spines of the tibia
- Dias disease: trochlea of the talus
- Dietrich disease: head of metacarpals
- Freiberg infraction: head of the second metatarsal
- Friedrich disease: medial clavicle
- Hass disease: humeral head
- Iselin disease: base of 5th metatarsal
- Kienböck disease: lunate
- Köhler disease: patella or navicular (children)
- Kümmell disease: vertebral body
- Legg-Calvé-Perthes disease: femoral head
- Mandl disease: greater trochanter
- Mauclaire disease: metacarpal heads
- Milch disease: ischial apophysis
- Mueller-Weiss disease: navicular (adult)
- Panner disease: capitellum of the humerus
- Pierson disease: symphysis pubis
- Preiser disease: scaphoid
- Sever disease: calcaneal epiphysis
- Siffert-Arkin disease: distal tibia
- Thiemann disease: base of phalanges
Clinical Features
The clinical features are dependent on the size and site of osteonecrosis. With small lesions at sites where there can be revascularisation, usually no clinical effect occurs. If there is a large area of osteonecrosis and revascularisation cannot occur then the area remains necrotic, leading to fracture, progressive collapse, joint incongruity, and joint destruction.
The most commonly affected regions are the femoral head, humeral head, talus, medial femoral condyle, and scaphoid.
Imaging
Plain Films
Plain radiographs are normal in early disease. Usually there is initial minor osteopenia, that is then followed by variable changes which can include patchy sclerosis and rim calcification. Gradually microfractures of the subchondral bone increase in number which isn't repaired. Eventually there is collapse of the articular surface and the crescent sign of osteonecrosis. Finally the cortex collapses and fragments, and secondary osteoarthritis is seen.
| Stage | Description | ||
|---|---|---|---|
| 0 | Asymptomatic, radiographically normal hip in a patient at risk | ||
| I | Symptomatic hip in which no radiographic change is evident | ||
| II | Patchy areas that are radiolucent and radiodense. The “crescent sign” represents the transition between stage II and stage III | ||
| III | Appearance of the crescent sign | ||
| IV | Collapse of femoral head | ||
| V | Narrowing and arthrosis of the joint |
In the talus, Hawkin's sign refers to subchondral radiolucency of the talar dome seen in AP view that occurs secondary to subchondral atrophy 6-8 weeks after talar neck fracture. Hawkin's sign indicates sufficient vascularity in the talus, whereas disruption of the blood supply to the talar dome results in absence of this sign indicating underlying avascular necrosis.
MRI
MRI is the most sensitive imaging modality. It shows changes well before plain films.[1]
- Reactive interface line: focal serpentine low signal line with fatty centre (most common appearance and first sign on MRI)
- Double line sign: T2WI serpentine peripheral/outer dark (sclerosis) and inner bright (granulation tissue) line is diagnostic (the line extends usually to the subchondral bone plate, which helps to differentiate it from subchondral fracture)
- Diffuse oedema: oedema is not an early sign; instead, studies show that oedema occurs in advanced stages and is directly correlated with pain
- Rim sign: osteochondral fragmentation
- Secondary degenerative change (i.e. osteoarthritis)
- On contrast-enhanced images non-viable marrow does not enhance
- In case of radiation necrosis, there is oedema or fatty replacement of the adjacent bone marrow (depending on the interval between the examination and radiotherapy)
Nuclear medicine
Bone scintigraphy is also quite sensitive (~85%) and is the second option after MRI. It is a choice when multiple sites of involvement must be assessed in patients with risk factors, such as sickle cell disease. The findings are different accordingly to the time of the scan:[1]
- early disease: often represented by a cold area likely representing the vascular interruption
- late disease: may show a "doughnut sign": a cold spot with surrounding high uptake ring (surrounding hyperaemia and adjacent synovitis)
Treatment
Treatment involves reducing load on the osteonecrotic part, and promoting revascularisation.
References
- Orthopaedic Basic Sciences 2nd Edition. Chapter 14 Bone Injury Regeneration and Repair
- ↑ 1.0 1.1 Osteonecrosis. Dr Daniel J Bell and Assoc Prof Frank Gaillard et al. Radiopaedia. From https://radiopaedia.org/articles/osteonecrosis-2

