Prostaglandins and Cyclooxygenase Enzymes
This article discusses the prostanoid biosynthesis pathway and cyclooxygenase enzymes. It is relevant to the topic of NSAIDs which is discussed in its own article.
Prostanoid Biosynthesis Pathway
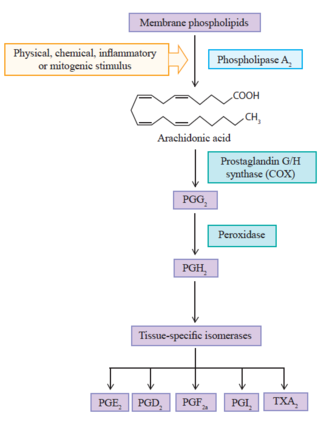
Biosynthesis starts with membrane phospholipids, present in the lipid bilayer of the cell membrane. The biosynthesis pathway has three main steps. (Figure 1)
Initially arachidonic acid is liberated from membrane phospholipids, by hydrolysis, by phospholipase A2 enzyme (PLA2). The second step involves the COX enzyme, through its cyclooxygenase site, which results in prostaglandin G2 (PGG2). The same COX enzyme converts PGG2 to prostaglandin H2 (PGH2) through the COX peroxidase catalytic site. One end of the COX enzyme has a membrane binding domain along with an epithelial growth factor domain, and the other end is the active enzymatic site.
PGH2 is converted to various kinds of prostanoids by tissue-specific isomerases. For example prostacyclin (PGI2), prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2a (PGF2a) and thromboxane A2 (TXA2).
Arachidonic acid can also be converted to leukotrienes by the enzyme lipoxygenase.
COX Enzymes
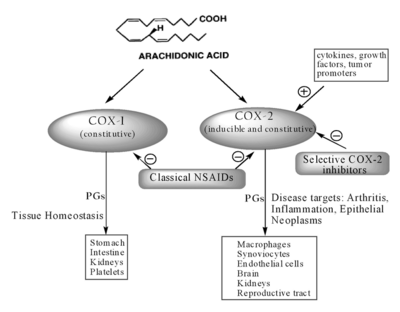
There are several different COX isoforms, but the most studied are COX-1 and COX-2. They differ only in the amino acid at site 523 with COX1 having isoleucin, and COX2 having valine. Both COX isoenzymes act on arachidonic acid, but the difference lies in genes that produce them and the factors that trigger those genes (Figure 2).
COX is also known as prostaglandin G/H synthase.
COX-1
COX-1 is a "housekeeping" enzyme that is constantly expressed in nearly all tissues such as platelets, gastrointestinal tract, kidney, endothelium, uterus, and brain.
It is involved in the mediation of physiological responses through maintaining a basal rate of prostanoids in the tissues. For example is involved in cytoprotection of the stomach, platelet aggregation, and facilitating blood flow through tissues especially the stomach and kidney.
COX-2
Traditionally COX-2 was thought to only be expressed by cells involved in inflammation such as macrophages, monocytes, and synoviocytes. The expression is triggered by tissue damage in order to promote repair. The production of COX-2 increases by 20 times in the presence of inflammatory stimuli, and so it is the primary enzyme that is responsible for the synthesis of prostanoids in acute and chronic pathological inflammatory states.
This is however an overly simplified view. COX-2 is not an exclusively proinflammatory inducible enzyme. It is basally expressed in many organs such as the ovary, uterus, brain, spinal cord, kidney, cartilage, bone, and the gastrointestinal tract. It plays a more complex physiological role than previously recognised. For example, in the kidney it is responsible for ensuring tubuloglomerular feedback.
The expression is regulated by several mediators e.g. lipopolysaccharide, proinflammatory cytokines, and growth factors. Glucocorticoids inhibit COX-2 expression.
Prostaglandins
Prostaglandin H2 (PGH2) has its own actions or can be converted to other prostaglandins such as PGE2, PGI2, PGD2, PGF2a, and TXA2.
- PGE2 produces fever, vasodilation with resulting lowering of blood pressure and promoting blood flow to the kidney and gastrointestinal tract, diuresis, and in the uterus it causes smooth muscle contraction.
- PGH2 increases blood pressure, i.e. it competes against PGE2.
- PGI2 has the same effects on the gastrointestinal tract as PG2. If fact all PGs have the same effect on the gastrointestinal tract.
- PGI2 inhibits the uterus, i.e. it competes against PGE2
- PGI2 inhibits platelet aggregation, i.e. it competes against thromboxane (TXA2). (Mnemonic THROMBOxane causes thrombosis, prostaCYCLIN keeps the blood cyclin')
- TXA2 promotes platelet aggregation.
Peripheral sensitisation: Prostaglandins regulate the sensitivity of polymodal nociceptors that are present in nearly all tissues. Many of these nociceptors can't be easily activated by physiological stimuli such as light pressure or slight increase in temperature. However, with tissue trauma and the release of prostaglandins, "silent" polymodal nociceptors are able to become excitable to pressure, temperature changes, and acidosis. This results in hyperalgesia and in some cases allodynia. This may be through effects on transient receptor potential vanilloid 1 (TRPV1) in sensory neurons, and tetrodotoxin-resistant sodium channels in dorsal root ganglia. Therefore, at least part of the analgesic action of COX inhibitors is thought to be due to the prevention of peripheral sensitisation.
Central sensitisation: COX-2 expression in the spinal cord may facilitate nociceptive transmission, with prostaglandins acting on the CNS to cause central hyperalgesia. There is widespread induction of COX-2 expression in spinal cord neurons and other regions of the CNS following peripheral inflammation. COX-2 is expressed in the dorsal horn and is upregulated briefly following trauma in the corresponding segment. Prostaglandin E2 has a role in central pain sensitisation. It directly depolarises wide dynamic range neurons into the deep dorsal horn, and reduces the inhibitory tone of the neurotransmitter glycine onto neurons in the superficial layers of the dorsal horn thereby disinhibiting nociceptive transmission.
See Also
References
- ↑ Goetz Moro, Marcella et al. Cyclooxygenase biology in renal function - literature review. Rev. colom. nefrol. 2017, vol.4, n.1 [cited 2021-08-21], pp.27-37. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S2500-50062017000100027&lng=en&nrm=iso
- ↑ Zarghi, Afshin, and Sara Arfaei. “Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships.” Iranian journal of pharmaceutical research : IJPR vol. 10,4 (2011): 655-83. cyclooxygenases (COX-1 and COX-2).

