Skeletal Muscle: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 68: | Line 68: | ||
Several of the constituent proteins of the costameres are defective in many muscular dystrophies, cardiomyopathies, and myopathies. | Several of the constituent proteins of the costameres are defective in many muscular dystrophies, cardiomyopathies, and myopathies. | ||
==See Also== | |||
*[[Myotendinous Junction]] | |||
== References == | == References == | ||
Revision as of 12:13, 10 August 2021
Striated muscle contains repeated functional units called sarcomeres which is seen as a series of bands along the muscle fibres on electron microscopy. There are two types of striated muscle: cardiac, and skeletal. This article focuses on skeletal striated muscle.
The force from cardiac and skeletal muscle contraction is generated by the shortening of individual sarcomeres. Force transmission can be directed either parallel (major force), or lateral to the long axis of the sarcomere.
Structure
Muscle cells are tubular in shape and are called muscle fibres or myofibres. Muscle fibres themselves contain numerous tubular myofibrils, which in turn are composed of repeating sections of sarcomeres. The sarcomeres is the basic structural unit of striated muscle, and under microscopy appear as alternating dark and light bands.
Components
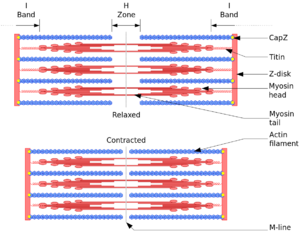
Sarcomeres contain contains three major compontents: the thick filament, thin filament, and titin.
- Thick filament: composed of myosin and other accessory proteins including myosin binding protein C. Myosin has a long, fibrous tail with a globular head, and binds to actin when the binding sites of actin are exposed by calcium ions. It also binds to ATP.
- Thin filament: assembled from actin monomers . Troponin and tropomyosin are also key components. Thin filaments connect to the Z-line, which is the border between adjacent sarcomeres
- Titin: This is a giant protein and it crosses from the Z-line to M-line. Its function is to act as a spring and ruler for the contractile apparatus. It is associated with another large protein called nebulin in skeletal muscle which is also thought to act as a molecular rule for the thin filaments.
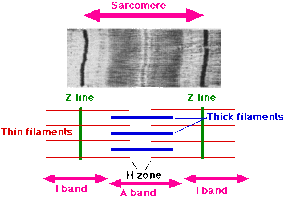
Bands
Sarcomeres give striated skeletal and cardiac muscle their striated appearance.
- Z-line (Z for zwischen meaning between in German): Sarcomeres are bound between two adjacent Z-lines. The Z-lines are dark and are located betwen the I-bands.
- I-band (I for isotropic): this zone consists of thin filaments that aren't superimposed by thick filaments
- A-band (A for anisotropic): The entire length of a single thick filament, containing both thick and thin filaments due to overlap.
- H-zone (H for heller meaning brighter in German): This is located within the A-band and is lighter in apperance. It contains thick filaments only.
- M-line (M for mittel meaning middle in German): This is within the H-zone
The sarcomeres align in reating units between two Z-lines. The Z-line is an important component of striated muscle.
- Appears dense on electron microscopy due to multiple highly ordered protein complexes.
- Is a hotspot for cellular signalling
- It also connects myofibrils to the sarcolemma membrane via the costamere (see below)
Contraction
Tropomyosin covers the myosin-binding sites of the actin muscles. In order for contraction to occur, tropomyosin is moved to uncover the actin binding sites. This occurs through calcium ions binding with troponin C molecules, which alters the structural composition of tropomyosin, leading to the cross-bridge binding sites to be revealed. troponin C molecules are dispersed throughout the tropomyosin protein.
The concentration of calcium within muscle cells is controlled by the sarcoplasmic reticulum, a unique form of endoplasmic reticulum in the sarcoplasm.
Muscle cells are stimulated when a motor neuron releases the neurotransmitter acetylcholine, which travels across the neuromuscular junction and binds to a post-synaptic nicotininc acetylcholine receptor. There is a change in the conformation of the receptor which allows sodium ions to flow into the muscle cell and an action potential is generated. The action potential travels along the T-tubules (T for transverse) towards the sarcoplasmic reticulum. At this site the depolarisation of the membrane from the action potential activates voltage-gated L-type calcium channels in the plasma membrane. Calcium flows inwards from these channels and activates ryanodine receptors which are also present on the sarcoplasmic reticulum. This mechanism is called calcium-induced calcium release.
The presence of calcium allows the myosin heads access to the actin cross-bridging sites as above, thereby allowing muscle contraction. Once calcium ions are pumped back into the sarcoplasmic reticulum, contraction ceases, and relaxation occurs.
During contraction, A-bands stay the same length, while the I-bands and H-bands shorten, allowing the Z lines to come closer together.
Rest
As stated above, at the resting state the myosin head is unable to access the cross-bridge binding sites on actin. in this state the myosin head is bound to an ATP molecule in a low-energy configuration.
- Low energy to high energy change: the myosin head hydrolyzes ATP into ADP and a phosphate ion. Some of the energy released changes the shape of the myosin head promoting it to a high-energy configuration
- High energy to low energy change: With binding to actin, there is release of ADP and phosphate ions from the myosin head, which changes it back to low energy. Myosin remains attached to actin in a state of rigor, until a new ATP binds to the myosin head. With ATP binding to myosin there is release of actin by cross-bridge dissociation. This ATP-associated myosin is then ready for another contraction cycle, starting with the hydrolysis of ATP.
The A-band is visible as dark transverse lines across myofibres; the I-band is visible as lightly staining transverse lines, and the Z-line is visible as dark lines separating sarcomeres at the light-microscope level.
Force Transmission
Long Axis Force Transmission
In long axis force transmission, the force is propagated along sarcomeres within the same fibre until its terminal end. Only 20-30% of the force generated by sarcomeres is propagated longitudinally.
Parallel Force Transmission through Costameres
Costameres are subsarcolemmal protein assemblies that are found circumferentially around striated muscle tissue. They are associated with the Z-line in peripheral myofibrils which forms a structural anchor and site for cell signalling.
Costameres function to
- Lateral force transmission: provide a physical coupling mechanism between peripheral myofibrils to the sarcolemma. They transmit contractile forces laterally from the sarcomeres across the sarcolemma to the extracellular matrix, and in turn to neighbouring muscle cells. In fact, the major force of sarcomeres is transduced laterally (70-80%). This allows for maintenance of uniform sarcomere length between adjacent contracting and resting muscle cells of different motor units within a particular skeletal muscle.
- Mechanical fortification: provide mechanical fortification in order to reduce stress on the lipid bilayer.
- Mechanical signalling: Orchestrate bidirectional mechanically related signalling between the Z-line and the extracellular matrix modulating myofibril growth and contraction.
The two most important protein complexes are the dystrophin–glycoprotein complex and the integrin–vinculin–talin complex.
Several of the constituent proteins of the costameres are defective in many muscular dystrophies, cardiomyopathies, and myopathies.
See Also
References
- Peter, Angela K et al. “The costamere bridges sarcomeres to the sarcolemma in striated muscle.” Progress in pediatric cardiology vol. 31,2 (2011): 83-88. doi:10.1016/j.ppedcard.2011.02.003