Small Fibre Neuropathy
| |
| Small Fibre Neuropathy |
|---|
Small fiber neuropathy (SFN) is a painful neuropathy involving selectively the Aδ and C nerve fibres. This leads to sensory, autonomic, or combined symptoms. Small fibre neuropathy (SFN) and large fibre neuropathy belong to a group of diseases known as peripheral neuropathies. Polyneuropathy refers to cases where either the large fibres are affected, or both the large and small fibres are affected. SFN refers to isolated small fibre involvement. About 40-50% of patient with fibromyalgia meet the diagnostic criteria for SFN.[2]
Nerve Anatomy
- See also: Basic Neurophysiology
Small fibre neuropathy results from damage to the nociceptive system. The majority of peripheral sensory nerves are unmyelinated C fibres and thinly myelinated Aδ fibres. Small fibre nerves also include the γ efferent fibres, and the preganglionic and postganglionic sympathetic C fibres. The large fibres in contrast are the Aβ afferents from the skin, muscle, and internal organs; and the α motor neurons.
- Characteristics of sensory nerve fibres
| Nerve fibre | Myelinated axons | Diameter (µm) | Conduction velocity (m/s) | Sensory information | Usefulness of electroneuromyography | Usefulness of QST |
|---|---|---|---|---|---|---|
| Aα | Yes | 13-20 | 80-120 | Proprioception, muscle spindle primary endings (Ia), golgi tendon organs (Ib) (and alpha motor neurons) | Yes (H reflex) | No |
| Aβ | Yes | 6-12 | 33-75 | Discriminative mechanoreception (touch, vibration), proprioception, pain modulation (block nociceptive information, allodynia in sensitisation) | Yes (sensory nerve conduction) | Yes |
| Aγ | Yes | 4-8 | 15-40 | Touch, pressure (and gamma motor neurons) | No | |
| Aδ | Thin | 1-5 | 3-30 | "rapid" pain, crude touch, pressure, temperature. AMH type I for rapid mechanical pain (high heat threshold >53C), AMH type II for rapid heat pain (lower heat threshold 43-47C). | No | Yes |
| C | No | 0.3-1.5 | 0.5-2.0 | "slow" pain, touch, pressure temperature (and postganglionic autonomic). Polymodal. | No | Yes |
| Receptor Type | Fibre Group | Modality |
|---|---|---|
| Cutaneous and subcutaneous mechanoreceptors | Touch | |
| Hair down | Aδ | Light stroking |
| Thermal receptors | Temperature | |
| Cold receptors | Aδ | Skin cooling (25°C) |
| Warm receptors | C | Skin warming (41°C) |
| Heat nociceptors | Aδ | Hot temperatures (>45°C) |
| Cold nociceptor | C | Cold temperatures (<5°C) |
| Nociceptors | Pain | |
| Mechanical | Aδ | Sharp, pricking pain |
| Thermal-mechanical | Aδ | Burning pain |
| Thermal-mechanical | C | Freezing pain |
| Polymodal | C | Slow, burning pain |
| Polymodal Muscle and Skeletal Mechanoreceptors | Limb proprioception | |
| Stretch-sensitive free endings | Aδ | Excess stretch or force |
Aetiology
Disorders known to contribute to SFN are listed below.[2] SFN is a generalised sensory nerve disease process with abnormalities in the structure and function of affected nerve fibres. It is histopathologically characterized by degeneration of small nerve fibre endings. The small unmyelinated nerves are affected.
| Category | Disease |
|---|---|
| Idiopathic (50%)[4] | |
| Metabolic |
|
| Immunological (up to 20%) |
|
| Toxic agents and medications |
|
| Genetic diseases (10-15%)[4][6] |
|
Pathophysiology
The pathophysiology is through to relate to dysregulation of voltage gated sodium channels with resultant hyperexcitability of dorsal root ganglion neurons. The sodium channel members found in small nerve fibres are Nav1.7 (SCN9A), Nav1.8 (SCN10A), and Nav1.9 (SCN11A). [4]
A demyelinating process is thought to be unlikely as the condition only affects small nerve fibres. Distal axonal loss or extraordinarily neuronal degeneration are potential mechanisms.[7] Normally sodium enters the cell and then calcium and sodium are pumped out through the coupled sodium-calcium exchanger (NCX). With dysregulation (such as from a pathogenic variant leading to reversal of sodium-calcium exchanger) of this balance there may be excess calcium in the cell leading to axonal degeneration.[8][9]
There may also be a mitochondrial role with trauma and ageing.[9] In small nerve fibres mitochondrial density is normally quite high. Dysfunctional mitochondria have been found in painful neuropathies especially in HIV, diabetes, and chemotherapy induced neuropathies.
There are four stages of neuropathy in unmyelinated nerve fibres
- Mild proliferation: Increase in number of isolated small Schwann cell projections which are irregular in shape.
- Fibre loss: decreased fibre numbers along with increased empty Schwann cells.
- Regeneration: Signs of regeneration of unmyelinated fibres along with fibre loss. There is an increase in the total number of unmyelinated fibres and small fibres with a diameter of below 0.8μm
- Advanced regeneration: Empty Schwann cells return to normal. There is only an increase of small nerve fibres with a diameter of below 0.8μm, and small isolated projections of Schwann cells.
SFN associated with diabetes may have a different underlying cause. This may involve axon swelling, and there can be progression to proximal large fibre or polyneuropathy.
Clinical Features
Patients have "positive" and "negative" symptoms related to Aδ and C fibre degeneration. Classical symptoms include burning pain, numbness, paraesthesia, and autonomic symptoms.
| Sensory symptoms |
|---|
|
| Dysautonomic symptoms |
|
History
Pain is the dominant symptom. The pattern is usually a length dependent polyneuropathy with pain most notable in the distal lower limbs, with the maximal pain in the feet. Most patients with the length-dependent pattern don't have pain in the head, torso, abdomen, groin, and buttocks.[11] The pain progressively ascends to more proximal sites, and later involves the upper limbs in a similar fashion of starting distally before spreading proximally. This pattern is most commonly seen in diabetics or those with neurotoxin exposure.[10]
However it can also present as a non-length dependent polyneuropathy (i.e. involving all limbs at the onset of symptoms) or an asymmetric mono-multiplex neuropathy (i.e. affecting one more sensory peripheral nerve).[10]
It can be clinically distinguished from erythromelalgia in that the erythema if it occurs is relatively mild, and there is no clear exacerbation/relief link to warmth/cold respectively.
In non-length dependent polyneuropathy there is proximal, diffuse, or patchy distribution of different parts of the body. This may include the face, mouth, scalp, trunk and upper limbs before or at the same time as the lower limbs are affected.[10] Non-length dependent features may be more likely to have an immune mediated disease association such as Sjogren's Syndrome and as a paraneoplastic phenomenon.[12]
In mono-multiplex neuropathy type presentations there may be burning mouth syndrome, notalgia paraesthetica, meralgia paraesthetica, vulvodynia, and Wartenberg neuropathy.[10]
Patients may also complain of allodynia or dysaesthesia type symptoms with intolerance to bed sheets, shoes, and clothes.
Autonomic symptoms are due to dysfunction of C-fibres and thinly myelinated Aδ fibres and can include sweating alterations, temperature dysregulation, dry mouth and eyes, orthostatic intolerance or tachycardia, erectile dysfunction.[10]
40-50% of patients with fibromyalgia meet the diagnostic criteria for SFN. In one small study, fibromyalgia patients with SFN were more likely to report dysautonomia and paraesthesias.[2]
Examination
- See also: Pain Oriented Sensory Testing
A thorough sensory examination is required and this includes multi-modal testing for allodynia as well as testing for hyperpathia.
Patients with SFN may have severe symptoms but a normal physical and neurological examination. However there may be positive and negative signs. Most affected patients have a combination of positive signs (e.g. hyperalgesia, and allodynia), and negative signs (e.g. diminished pin prick and temperature sense). The majority of peripheral sensory nerves are unmyelinated C fibres and thinly myelinated Aδ fibres. There is no clear way of diagnosing pathology in these fibres.
Proprioception, light touch, and vibration sense may be normal. Some patients may have decreased pinprick, decreased thermal sensation, hyperalgesia in the affected region, and slightly decreased vibratory sense.
Diagnostic Classification
There is no gold standard for diagnosis. One suggestion is making the diagnosis based on 2/3 abnormal findings of clinical features, quantitative sensory testing, and skin biopsy; or quantitative sudomotor axon-reflex test (QSART) as an alternative to skin biopsy.[7]
The following diagnostic criteria have been published.
| Presence of at least two of the following: | Absence of the following: |
|---|---|
|
|
| Possible SFN | symptoms or clinical signs of small fibre damage |
|---|---|
| Probable SFN | symptoms or clinical signs of small fibre damage and normal sural nerve conduction studies |
| Definite SFN | symptoms or clinical signs of SFN-damage, normal sural nerve conduction studies and decreased intra-epidermal nerve fibre density (IENFD) and/or abnormal quantitative sensory testing (QST) thermal thresholds |
Investigations
See Raasing et al for an in depth open access review of diagnostic methods.[7]
Screen for Systemic Causes
A large cohort study used the following tests to find a cause in around 50% of patients.[15]
Blood tests: FBC, UEC, LFTs, HbA1c, TSH, Vitamin B12, Vitamin B6 (toxicity), Vitamin B1, celiac serology, ANA/ENA, serum protein electrophoresis, HIV, Alpha-galactosidase A activity (Fabry disease), ?ferritin (haemochromatosis), ?calcium (sarcoidosis)
Urine: urine lysosomal globotriasylceramide (Fabry disease)
Genetic testing: SCN9A, SCN109A, and SCN11A.
CXR: to investigate for sarcoidosis.
Also screen for alcohol abuse.
Questionnaires
These tools are obviously limited by their subjective nature. Neuropathic pain can be diagnosed using the Douleur Neuropathique 4 (DN4), and other similar instruments. There are multiple specific questionnaires for small fibre neuropathy, with validation done in different populations.[7]
Nerve Conduction Studies
Standard electrophysiologic testing is typically normal in SFN as the pathology lies in the small unmyelinated nerve fibres. However nerve conduction studies can be used to exclude large fibre involvement. This is recommended over the other QST procedures for testing large fibres.
Testing for Sensory Symptoms
Quantitative Sensory Testing
Quantitative sensory testing (QST) is an extension of the physical examination, and can be used to diagnose peripheral nervous system disorders. Testing involves thermal, pressure, vibration, and electrical stimulation, with the full battery of tests involving 13 parameters. The results are compared to normative values.
Due to the time consuming nature of full QST, specific thermal threshold testing (TTT) can be used to test small fibre function instead[16]. In TTT an electrode is used that has a baseline temperature of 32C and this can increase up to 50C or down to 0C. There are two testing methods.
- Method of limits (reaction time dependent): start at the baseline temperature and increase or decrease the temperature. The test button is pressure twice, first when the temperature change is felt, and second when it becomes painful.
- Method of levels (reaction time independent): there are two buttons for yes or no. For each stimulus the patient is asked whether the thermode becomes colder or not. There is no pain threshold testing in method of levels, only thermal detection.
The feet are tested first because they are more commonly abnormal than the hands. If the feet have abnormal thresholds then the hands don't need to be tested. However if the feet are normal then the hands need to be tested. Most studies seem to use the Medoc devices which appear to be quite expensive.
Normative values for TTT: normal temperature detection thresholds lay above 41C (C fibres) and below 25C (Aδ fibres), and temperature pain thresholds lay above 45C (Aδ fibres) and below 5C (C fibres).
There are some important limitations to QST in general. There is great inter-observer variability and a lack of world-wide standardisation with iportant differences in methods between normative values.. Either central or peripheral nervous system abnormalities can cause the same deficit. QST requires good cognitive function and good conscious patient reactions.[2] There are numerous other factors that can affect the results such as environmental conditions, gender, instructions, habituation, and motor performance.[7]
The Quantitative Sudomotor Axon Reflex Test (QSART) is used to evaluate autonomic function, in particular the peripheral sympathetic cholinergic nervous system. It measures the response of the autonomic sudomotor nerves. Iontophoresis is used to introduce acetylcholine into the skin, which stimulates the sweat glands. The volume of sweat produced is measured. Some patients with SFN have increased sweat production. [2]
Skin Biopsy
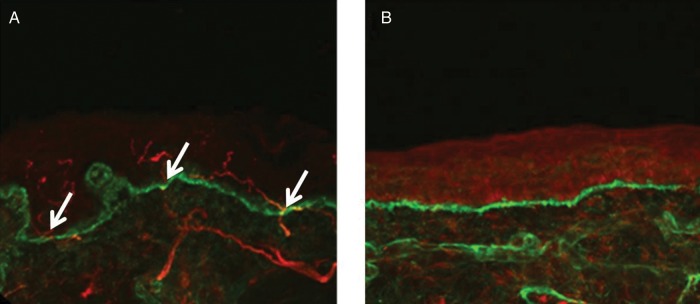
Sural nerve biopsy may be only minimally abnormal, or even normal.[2] It is performed using a 3mm punch under sterile technique. It can be taken from any body part, but the standard biopsy is 10cm above the lateral malleolus in the region of sural nerve innervation to enable evaluation of the loss of the most distal sensory endings that are typical of length dependent axonal neuropathy. Another site is the upper thigh (20cm distal to the iliac spine). The guidelines on performing the skin biopsy were established by the European Federation of Neurological Societies in 2005. The biopsy should be 3mm thick to enable assessment of both the epidermis and dermis. Suturing is not required. Risks include infection, tenderness at biopsy site, delayed healing, bleeding, allergy.[2]
The biopsy sample is stained immunohistochemically with antibodies against protein gene product 9.5 (PGP 9.5). This protein is a marker for peripheral nerve fibres and neuroendocrine cells. Most cutaneous nerve fibres are unmyelinated, but in the dermis of hair skin 10% are small diameter myelinated fibres (A-delta fibres). A fibre count is done, and single axons are counted that cross or originate at the epidermal-dermal junction. The result is the Epidermal Nerve Fibre Density (ENFD). Reduced ENFD has a 90% specificity and 82.6% sensitivity for small fibre neuropathy.[2][17]
In New Zealand the PGP9.5 stain doesn't appear to be available, but it may be possible to request an epidermal nerve fibre density assessment through other stains. It is viewed as a controversial investigation by pathologists in New Zealand.
Corneal Confocal Microscopy
This is a non-invasive test as an alternative to skin biopsy. It is comparable to skin biopsy in that you are looking for reduced nerve fibre density in SFN.[18]
Testing for Autonomic Symptoms
There are a variety of tests for autonomic symptoms. The most applicable for clinical care are cardiovagal tests, water induced skin wrinkling, neuropad, sudoscan, pupillometry, and bladder function tests.
Water-induced and EMLA-induced Skin Wrinkling
The patient places both hands in a bath of water heated to 40 degrees. The wrinkling is graded for the second to fifth fingers. Grade 0: wrinkling absent; grade 1: slight wrinkling and the fingertip is not smooth; grade 2: two or less lines of shallow wrinkling on the fingertip; grade 3: three or more lines of clear cut lines of wrinkling on the fingertip; grade 4: wrinkling completely distorts the pulp of the fingertip. The grade is averaged across fingers.
EMLA can be used as an alternative to induce skin wrinkling. Apply 1g to the distal digit tips of the 2nd to 5th fingers, and leave it to soak into the skin for 30 minutes after covering with Tegaderm.
In both cases, with sympathetic nerve dysfunction, there is less wrinkling seen due to prevention of vasovonstriction. Studied in patients with sensory neuropathy, the diagnostic accuracy depends on the cut off of the comparison standard of reduced intraepithelial nerve fibre density. Overall both tests have good positive predictive values, but poor negative predictive values. There was no correlation between grade of wrinkling and nerve density. For wrinkling grading, a score of 12 or more for each hand was used as the cut off for normal, and a difference of 4 or more difference per hand was used as a cut-off for a different score.[19]
Cardiovagal Tests
One abnormal cardiovagal test is possible cardiovascular autonomic neuropathy, two positive tests is definite, and severe is defined as orthostatic hypotension in addition to definite.
Heart rate response to valsalva (parasympathetic) is a test where the RR interval is measured on ECG to determine heart rate variability. There are five phases: ; phase (0) deep inspiration, phase (I) onset of strain, phase (II) continued strain, phase (III) release, phase (IV) recovery. The ratio is calculated by taking the shortest RR interval in phase II and dividing it by the longest in phase IV. An abnormal result is a lack of bradycardia during phase IV and a decreased valsalva ratio.
Heart rate response to postural change (parasympathetic) is where the patient changes from supine to upright and the heart rate is measured. A normal result is where the heart rate increases initially and then reduces. The 30:15 ratio is calculated, taking the bradycardia at 30 seconds and dividing it by the tachycardia at 15 seconds. Normally the heart rate increases by 10 seconds. In autonomic dysfunction there is no bradycardia.
Heart rate response to deep inspiration (parasympathetic) is looking for variability of the amplitude of individual electrical complexes on ECG with deep inspiration.
Blood pressure response to valsalva (sympathetic): In autonomic dysfunction there is no overshoot in blood pressure and bradycardia reflex.
Blood pressure response to postural change (sympathetic): The blood pressure should increase by 10mmHg after 1-2 minutes.
Genetic disease
The following genetic tests have been proposed in some cases.[10]
- Sodium channelopathy - SCN9A, SCN10A, SCN11A genes (gain of function mutations). Most variants area associated with distal pain.
- Familial amyloidosis - Transthyretin gene
- Fabry disease - Enzymatic assay for alpha-Gal A activity / Genetic test of alpha-Gal A (GLA)
Of the above the sodium channelopathies are the most common. Unfortunately there is no great difference between those with pathogenic variants and those with no variant. The only association is that those with a channelopathy are more likely to have a family history and have pain aggravated by warmth.[6]
Management
There is no known cure for SFN and the results of medication are usually disappointing with a lot of side effects. Standard neuropathic pain medications can be trialled such as gabapentinoids, topiramate, TCAs, and SNRIs. The combination of a TCA and gabapentinoid may be more effect than monotherapy.[2] Opioids are not recommended, but weak opioids such as codeine or tramadol can be considered for short periods of use only. Topical lidocaine or capsaicin has been used.
In those with a SCN9A pathogenic variant, lacosamide can be used with modest effect, with 40% feeling very much improved vs 12% with placebo in a small RCT.[20]
Resources
Euro-NMD Painful Neuropathies Lecture
See Also
References
Papers of particular interest have been highlighted as: ◆ of special interest ◆◆ of outstanding interest
- ↑ Themistocleous, Andreas C.; Ramirez, Juan D.; Serra, Jordi; Bennett, David L. H. (2014-12). "The clinical approach to small fibre neuropathy and painful channelopathy". Practical Neurology. 14 (6): 368–379. doi:10.1136/practneurol-2013-000758. ISSN 1474-7766. PMC 4251302. PMID 24778270. Check date values in:
|date=(help) - ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Maslinska et al. Small fibre neuropathy as a part of fibromyalgia or a separate diagnosis? Int. J. Clin. Rheumatol. (2018) 13(6), 353-359. Full Text
- ↑ Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. 4th ed. Appleton & Lange; 2000
- ↑ 4.0 4.1 4.2 4.3 4.4 de Greef, B. T. A.; Hoeijmakers, J. G. J.; Gorissen-Brouwers, C. M. L.; Geerts, M.; Faber, C. G.; Merkies, I. S. J. (2018-02). "Associated conditions in small fiber neuropathy - a large cohort study and review of the literature". European Journal of Neurology. 25 (2): 348–355. doi:10.1111/ene.13508. ISSN 1468-1331. PMC 5814938. PMID 29112785. Check date values in:
|date=(help) - ↑ Cazzato, Daniele et al. “Small fiber neuropathy is a common feature of Ehlers-Danlos syndromes.” Neurology vol. 87,2 (2016): 155-9. doi:10.1212/WNL.0000000000002847
- ↑ 6.0 6.1 Eijkenboom, Ivo; Sopacua, Maurice; Hoeijmakers, Janneke G. J.; de Greef, Bianca T. A.; Lindsey, Patrick; Almomani, Rowida; Marchi, Margherita; Vanoevelen, Jo; Smeets, Hubertus J. M.; Waxman, Stephen G.; Lauria, Giuseppe (2019-03). "Yield of peripheral sodium channels gene screening in pure small fibre neuropathy". Journal of Neurology, Neurosurgery, and Psychiatry. 90 (3): 342–352. doi:10.1136/jnnp-2018-319042. ISSN 1468-330X. PMID 30554136. Check date values in:
|date=(help) - ↑ 7.0 7.1 7.2 7.3 7.4 Raasing, Lisette R M et al. “Current View of Diagnosing Small Fiber Neuropathy.” Journal of neuromuscular diseases vol. 8,2 (2021): 185-207. doi:10.3233/JND-200490
- ↑ Persson, Anna-Karin; Liu, Shujun; Faber, Catharina G.; Merkies, Ingemar S. J.; Black, Joel A.; Waxman, Stephen G. (2013-01). "Neuropathy-associated Nav1.7 variant I228M impairs integrity of dorsal root ganglion neuron axons". Annals of Neurology. 73 (1): 140–145. doi:10.1002/ana.23725. ISSN 1531-8249. PMID 23280954. Check date values in:
|date=(help) - ↑ 9.0 9.1 Persson, Anna-Karin; Hoeijmakers, Janneke G. J.; Estacion, Mark; Black, Joel A.; Waxman, Stephen G. (2016-05-01). "Sodium Channels, Mitochondria, and Axonal Degeneration in Peripheral Neuropathy". Trends in Molecular Medicine (in English). 22 (5): 377–390. doi:10.1016/j.molmed.2016.03.008. ISSN 1471-4914. PMID 27085813.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 ◆◆ Devigili, Grazia; Cazzato, Daniele; Lauria, Giuseppe (2020-09-01). "Clinical diagnosis and management of small fiber neuropathy: an update on best practice". Expert Review of Neurotherapeutics. 20 (9): 967–980. doi:10.1080/14737175.2020.1794825. ISSN 1473-7175. PMID 32654574.
- ↑ ◆ Brouwer, Brigitte A.; Kuijk, Sander M. J. van; Bouwhuis, Anne; Faber, Catharina G.; Kleef, Maarten van; Merkies, Ingemar S. J.; Hoeijmakers, Janneke G. J. (2019-06-01). "The Pain Dynamics of Small Fiber Neuropathy". The Journal of Pain (in English). 20 (6): 655–663. doi:10.1016/j.jpain.2018.11.009. ISSN 1526-5900. PMID 30529697.
- ↑ Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve. 2012 Jan;45(1):86-91. doi: 10.1002/mus.22255. PMID: 22190313.
- ↑ Devigili, Grazia; Tugnoli, Valeria; Penza, Paola; Camozzi, Francesca; Lombardi, Raffaella; Melli, Giorgia; Broglio, Laura; Granieri, Enrico; Lauria, Giuseppe (2008-07). "The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology". Brain: A Journal of Neurology. 131 (Pt 7): 1912–1925. doi:10.1093/brain/awn093. ISSN 1460-2156. PMC 2442424. PMID 18524793. Check date values in:
|date=(help) - ↑ Tesfaye, Solomon; Boulton, Andrew J. M.; Dyck, Peter J.; Freeman, Roy; Horowitz, Michael; Kempler, Peter; Lauria, Giuseppe; Malik, Rayaz A.; Spallone, Vincenza; Vinik, Aaron; Bernardi, Luciano (2010-10). "Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments". Diabetes Care. 33 (10): 2285–2293. doi:10.2337/dc10-1303. ISSN 1935-5548. PMC 2945176. PMID 20876709. Check date values in:
|date=(help) - ↑ ◆
de Greef, B. T. A.; Hoeijmakers, J. G. J.; Gorissen-Brouwers, C. M. L.; Geerts, M.; Faber, C. G.; Merkies, I. S. J. (2018-02). "Associated conditions in small fiber neuropathy - a large cohort study and review of the literature". European Journal of Neurology. 25 (2): 348–355. doi:10.1111/ene.13508. ISSN 1468-1331. PMC 5814938. PMID 29112785. Check date values in:
|date=(help) - ↑ Bakkers, Mayienne; Faber, Catharina G.; Reulen, Jos P. H.; Hoeijmakers, Janneke G. J.; Vanhoutte, Els K.; Merkies, Ingemar S. J. (2015-06). "Optimizing temperature threshold testing in small-fiber neuropathy". Muscle & Nerve. 51 (6): 870–876. doi:10.1002/mus.24473. ISSN 1097-4598. PMID 25290248. Check date values in:
|date=(help) - ↑ Lauria et al.. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. European journal of neurology 2010. 17:903-12, e44-9. PMID: 20642627. DOI.
- ↑ Kalteniece, Alise; Ferdousi, Maryam; Azmi, Shazli; Mubita, Womba M.; Marshall, Andrew; Lauria, Giuseppe; Faber, Catharina G.; Soran, Handrean; Malik, Rayaz A. (2020-02-25). "Corneal confocal microscopy detects small nerve fibre damage in patients with painful diabetic neuropathy". Scientific Reports. 10 (1): 3371. doi:10.1038/s41598-020-60422-7. ISSN 2045-2322. PMC 7042367. PMID 32099076.
- ↑ Wilder-Smith EP, Guo Y, Chow A. Stimulated skin wrinkling for predicting intraepidermal nerve fibre density. Clin Neurophysiol. 2009 May;120(5):953-8. doi: 10.1016/j.clinph.2009.03.011. Epub 2009 Apr 16. PMID: 19375384.
- ↑ de Greef, Bianca T. A.; Hoeijmakers, Janneke G. J.; Geerts, Margot; Oakes, Mike; Church, Tim J. E.; Waxman, Stephen G.; Dib-Hajj, Sulayman D.; Faber, Catharina G.; Merkies, Ingemar S. J. (2019-02-01). "Lacosamide in patients with Nav1.7 mutations-related small fibre neuropathy: a randomized controlled trial". Brain: A Journal of Neurology. 142 (2): 263–275. doi:10.1093/brain/awy329. ISSN 1460-2156. PMID 30649227.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,


