Smoking and Chronic Pain: Difference between revisions
No edit summary |
|||
| Line 5: | Line 5: | ||
Tobacco smoke contains nicotine and over 4000 other compounds. Nicotine plays a role in pain-related pathophysiology. | Tobacco smoke contains nicotine and over 4000 other compounds. Nicotine plays a role in pain-related pathophysiology. | ||
Nicotine binds to nicotinic acetylcholine receptors (nAChRs) which are found throughout the central and peripheral nervous systems and are involved in the physiology of arousal, sleep, anxiety, cognition, and pain. | Nicotine binds to nicotinic acetylcholine receptors (nAChRs) which are found throughout the central and peripheral nervous systems and are involved in the physiology of arousal, sleep, anxiety, cognition, and pain. Nicotine also binds to opioid receptors. | ||
=== Short Term Use === | === Short Term Use === | ||
| Line 29: | Line 29: | ||
In the short term nicotine withdrawal worsens pain, however in the long term cessation is likely to be beneficial.<ref name=":1" /> Some of the harmful effects may not be reversible. The prevalence rates of chronic pain in ex-smokers is higher than nonsmokers. However chronic pain is more severe in smokers than nonsmokers and ex-smokers when looking at pain frequency, intensity, duration, and number of painful sites.<ref>{{Cite journal|last=Ditre|first=Joseph W.|last2=Brandon|first2=Thomas H.|last3=Zale|first3=Emily L.|last4=Meagher|first4=Mary M.|date=2011-11|title=Pain, nicotine, and smoking: research findings and mechanistic considerations|url=https://pubmed.ncbi.nlm.nih.gov/21967450|journal=Psychological Bulletin|volume=137|issue=6|pages=1065–1093|doi=10.1037/a0025544|issn=1939-1455|pmc=3202023|pmid=21967450}}</ref> | In the short term nicotine withdrawal worsens pain, however in the long term cessation is likely to be beneficial.<ref name=":1" /> Some of the harmful effects may not be reversible. The prevalence rates of chronic pain in ex-smokers is higher than nonsmokers. However chronic pain is more severe in smokers than nonsmokers and ex-smokers when looking at pain frequency, intensity, duration, and number of painful sites.<ref>{{Cite journal|last=Ditre|first=Joseph W.|last2=Brandon|first2=Thomas H.|last3=Zale|first3=Emily L.|last4=Meagher|first4=Mary M.|date=2011-11|title=Pain, nicotine, and smoking: research findings and mechanistic considerations|url=https://pubmed.ncbi.nlm.nih.gov/21967450|journal=Psychological Bulletin|volume=137|issue=6|pages=1065–1093|doi=10.1037/a0025544|issn=1939-1455|pmc=3202023|pmid=21967450}}</ref> | ||
Pain intensity starts to decrease after about 3 weeks of abstinence. However in the surgical literature, postoperative pain is worse than nonsmokers even if abstinence occurs 3 weeks before surgery. Patience is needed to see the benefits of smoking cessation on pain. | Pain intensity starts to decrease after about 3 weeks of abstinence. However in the surgical literature, postoperative pain is worse than nonsmokers even if abstinence occurs 3 weeks before surgery. Patience is needed to see the benefits of smoking cessation on pain. | ||
= | Patients with pain are often refractory to smoking cessation treatment. However overall smoking cessation in the long term is highly recommended in individuals with chronic pain. It prevents exacerbation of underlying causes of pain. Several studies have found that patients who quit smoking have improvements in pain and improved response to various treatments. It is imperative to address underlying psychological issues.<ref name=":1" /> | ||
Vaping is also negatively impacts pain perception | |||
Vaping is not a good alternative because it also negatively impacts pain perception due to containing which increases pain sensitivity.<ref name=":1" /> | |||
Prescription options to assist with smoking cessation include nicotine replacement therapy, bupropion, nortriptyline, and varenicline. As of December 2022 varenicline has been unavailable in New Zealand for many months. Bupropion and nortriptyline can be good options due to their mild analgesic effects.<ref name=":1" /><ref>{{Cite journal|last=Semenchuk|first=M. R.|last2=Sherman|first2=S.|last3=Davis|first3=B.|date=2001-11-13|title=Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain|url=https://pubmed.ncbi.nlm.nih.gov/11706096|journal=Neurology|volume=57|issue=9|pages=1583–1588|doi=10.1212/wnl.57.9.1583|issn=0028-3878|pmid=11706096}}</ref> | |||
Exercise should be promoted as a healthier alternative to analgesia through [[Exercise Induced Hypoalgesia|exercise induced hypoalgesia]]. | |||
== Opioid Use == | == Opioid Use == | ||
Revision as of 12:16, 4 December 2022
The prevalence of smoking in those with chronic pain is up to double that of the general population. In the short term smoking is an analgesic, however in the long-term it is deleterious for pain as it exacerbates nociceptive, neuropathic, and psychosocial pain.[1] Smokers have higher pain intensities, number of painful areas, levels of disability, and opioid use to nonsmokers. [2]
Pathophysiology
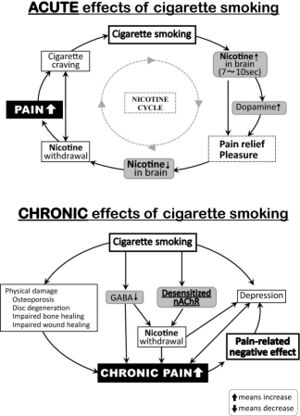
Tobacco smoke contains nicotine and over 4000 other compounds. Nicotine plays a role in pain-related pathophysiology.
Nicotine binds to nicotinic acetylcholine receptors (nAChRs) which are found throughout the central and peripheral nervous systems and are involved in the physiology of arousal, sleep, anxiety, cognition, and pain. Nicotine also binds to opioid receptors.
Short Term Use
Cigarette smoking results in increased brain concentrations of nicotine within 7-10 seconds. It binds to nAChRs in the midbrain as well as in the peripheral nervous system. nAChR activation results in the release of noradrenaline, endogenous opioids, dopamine, and other neurotransmitters. This results in the feelings of euphoria and analgesia. The analgesic effect is from activation of the descending pain modulatory pathways and inhibition of afferent input to the dorsal horn.[1]
A meta-analysis for intranasal or transdermal nicotine found a small beneficial analgesic effect when used in the short-term for post-operative pain.[3]
Long Term Use
However inhaled nicotine has a half-life of approximately 30 minutes. This leads to a rapid decrease in the levels of the above neurotransmitters. This then leads to increased withdrawal, greater pain intensity, and cravings. Increased pain sensitivity occurs because long term use of nicotine results in neuroplastic changes with nAChR desensitisation. The only way to overcome this would be to smoke constantly, which is not typically possible, and it would have other deleterious effects.
Therefore with use in the longer term pain is worsened, and the short term analgesic effects are outweighed by increased pain sensitivity from nicotine withdrawal due to rapid brain elimination of nicotine. There may also be reduced endogenous opioid release, blunting of the hypothalamic-pituitary-adrenal axis during stress, and altered connectivity of the basal ganglia.
Furthermore smoking has other deleterious effects on the musculoskeletal system. It increases the risk of disc degeneration, fractures, delayed tissue healing, and osteoporosis. From a psychosocial perspective it can further intensify depressive and anxiety symptoms and cause sleep disturbance. Smokers are also more likely to abuse alcohol, other substances, and have suicidal ideation.
In rat models nicotine exposure results in improved pain thresholds after 1 to 3 weeks of exposure. However after 6 weeks of use pain thresholds are worsened. Chronic exposure leads to decreased pain thresholds in a neuropathic pain model following withdrawal.
Effect on Pain Interventions
In the surgical setting, smokers have a higher incidence of inadequate post-operative pain control than nonsmokers and higher rates of chronic post-surgical pain regardless of the type of surgery (including arthroplasty and spinal surgery), as well as higher risk of chronic opioid use post surgery. Smokers also have poorer outcomes with nerve blocks and spinal cord simulators.[1]
Smokers have poorer outcomes in MDT pain management programmes as well as lower rates of return to work.[1]
Smoking Cessation
In the short term nicotine withdrawal worsens pain, however in the long term cessation is likely to be beneficial.[1] Some of the harmful effects may not be reversible. The prevalence rates of chronic pain in ex-smokers is higher than nonsmokers. However chronic pain is more severe in smokers than nonsmokers and ex-smokers when looking at pain frequency, intensity, duration, and number of painful sites.[4]
Pain intensity starts to decrease after about 3 weeks of abstinence. However in the surgical literature, postoperative pain is worse than nonsmokers even if abstinence occurs 3 weeks before surgery. Patience is needed to see the benefits of smoking cessation on pain.
Patients with pain are often refractory to smoking cessation treatment. However overall smoking cessation in the long term is highly recommended in individuals with chronic pain. It prevents exacerbation of underlying causes of pain. Several studies have found that patients who quit smoking have improvements in pain and improved response to various treatments. It is imperative to address underlying psychological issues.[1]
Vaping is not a good alternative because it also negatively impacts pain perception due to containing which increases pain sensitivity.[1]
Prescription options to assist with smoking cessation include nicotine replacement therapy, bupropion, nortriptyline, and varenicline. As of December 2022 varenicline has been unavailable in New Zealand for many months. Bupropion and nortriptyline can be good options due to their mild analgesic effects.[1][5]
Exercise should be promoted as a healthier alternative to analgesia through exercise induced hypoalgesia.
Opioid Use
Not only is opioid use more common in smokers compared to non-smokers, there is also an increased quantity of opioid use per individual. Even with higher use of opioids pain control is still worse than non-smokers. This association holds when controlling for confounding factors. It is also more difficult for smokers who use opioids to quite smoking. The analgesic effect of opioids is enhanced by supraspinal nicotinic acetylcholine receptors.[2]
Specific Conditions
Smoking is harmful in a variety of pain conditions including but not limited to headache disorders, low back pain, fibromyalgia, inflammatory arthritis, neuropathic pain, and cancer.
Low Back Pain
Smoking is a risk factor for disc degeneration and the development of chronic low back pain. The prevalence of smoking in this group reported to be between 16-40%.[2] One large cohort study of over 70 thousand Canadians found a clear relationship between smoking and chronic low back pain risk. The prevalence was 23.3% in daily smokers compared to 15.7% in non-smokers. The association was stronger amongst younger individuals and the effect was dose dependent.[6] The rates of low back pain are higher even in passive smokers.[1]
Fibromyalgia
The rate of smoking amongst patients with fibromyalgia is higher than the general population. For example one study of 1566 patients reported a smoking prevalence of 38.7% in those with fibromyalgia compared to 24.7% of patients without fibromyalgia.[7]
Rheumatoid Arthritis
Rheumatoid arthritis is approximately twice as more prevalent in smokers compared to nonsmokers, and four times more prevalent for rheumatoid-factor positive rheumatoid arthritis.[8]
Neuropathic Pain
The rates of sciatica and postherpetic neuralgia are approximately twice that of nonsmokers.[1]
Cancer
Smoking is harmful in those with cancer for several reasons. It causes increased pain sensitivity, increased need for opioids, increased rates of chemotherapy induced peripheral neuropathy, and [1]increased pain with radiation.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Iida, Hiroki; Yamaguchi, Shigeki; Goyagi, Toru; Sugiyama, Yoko; Taniguchi, Chie; Matsubara, Takako; Yamada, Naoto; Yonekura, Hiroshi; Iida, Mami (2022-12). "Consensus statement on smoking cessation in patients with pain". Journal of Anesthesia. 36 (6): 671–687. doi:10.1007/s00540-022-03097-w. ISSN 1438-8359. PMC 9666296. PMID 36069935. Check date values in:
|date=(help) - ↑ 2.0 2.1 2.2 Orhurhu, Vwaire J.; Pittelkow, Thomas P.; Hooten, W. Michael (2015). "Prevalence of smoking in adults with chronic pain". Tobacco Induced Diseases. 13 (1): 17. doi:10.1186/s12971-015-0042-y. ISSN 2070-7266. PMC 4504349. PMID 26185492.
- ↑ Matthews, Annette M.; Fu, Rongwei; Dana, Tracy; Chou, Roger (2016-01-12). "Intranasal or transdermal nicotine for the treatment of postoperative pain". The Cochrane Database of Systematic Reviews. 2016 (1): CD009634. doi:10.1002/14651858.CD009634.pub2. ISSN 1469-493X. PMC 8729826. PMID 26756459.
- ↑ Ditre, Joseph W.; Brandon, Thomas H.; Zale, Emily L.; Meagher, Mary M. (2011-11). "Pain, nicotine, and smoking: research findings and mechanistic considerations". Psychological Bulletin. 137 (6): 1065–1093. doi:10.1037/a0025544. ISSN 1939-1455. PMC 3202023. PMID 21967450. Check date values in:
|date=(help) - ↑ Semenchuk, M. R.; Sherman, S.; Davis, B. (2001-11-13). "Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain". Neurology. 57 (9): 1583–1588. doi:10.1212/wnl.57.9.1583. ISSN 0028-3878. PMID 11706096.
- ↑ Alkherayf, Fahad; Agbi, Charles (2009-10-01). "Cigarette smoking and chronic low back pain in the adult population". Clinical and Investigative Medicine. Medecine Clinique Et Experimentale. 32 (5): E360–367. doi:10.25011/cim.v32i5.6924. ISSN 1488-2353. PMID 19796577.
- ↑ Goesling, Jenna; Brummett, Chad M.; Meraj, Taha S.; Moser, Stephanie E.; Hassett, Afton L.; Ditre, Joseph W. (2015-07). "Associations Between Pain, Current Tobacco Smoking, Depression, and Fibromyalgia Status Among Treatment-Seeking Chronic Pain Patients". Pain Medicine (Malden, Mass.). 16 (7): 1433–1442. doi:10.1111/pme.12747. ISSN 1526-4637. PMC 4765172. PMID 25801019. Check date values in:
|date=(help) - ↑ Sugiyama, D.; Nishimura, K.; Tamaki, K.; Tsuji, G.; Nakazawa, T.; Morinobu, A.; Kumagai, S. (2010-01). "Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies". Annals of the Rheumatic Diseases. 69 (1): 70–81. doi:10.1136/ard.2008.096487. ISSN 1468-2060. PMID 19174392. Check date values in:
|date=(help)

