Superior Cluneal Nerve Entrapment: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 118: | Line 118: | ||
[[Category:Lumbar Spine Conditions]] | [[Category:Lumbar Spine Conditions]] | ||
[[Category:Peripheral Nerve Entrapments]] | [[Category:Peripheral Nerve Entrapments]] | ||
[[Category:Pelvis, Hip and Thigh]] | [[Category:Pelvis, Hip and Thigh Conditions]] | ||
Revision as of 09:43, 3 March 2022
| Superior Cluneal Nerve Entrapment | |
|---|---|
| Synonym | Cluneal nerve entrapment, cluneal neuralgia, clunealgia |
| Epidemiology | <8% of chronic low back pain, unproven |
| Pathophysiology | Repetitive friction of SCN under the thoracolumbar fascia, or rarely constriction in the osteofibrous tunnel. |
| Risk Factors | Thoracolumbar site fractures at the origin of the SCN |
| Diagnosis | focal iliac crest tenderness, reproduction of symptoms with pressure, and pain reduction with block. |
| DDX | Sacroiliac joint pain, referred lumbar spine pain |
| Validity | Plausible but not proven. |
| Treatment | Repeated local anaesthetic injections, SCN or MCN decompression |
The cluneal nerves are a group of pure sensory nerves (superior, medial or middle, and inferior) that provide sensory supply to the buttocks. This article deals with superior cluneal nerve entrapment. It is contentious whether cluneal nerve entrapment is an important cause of chronic low back pain. See also the less common middle cluneal nerve entrapment and inferior cluneal nerve entrapment.
Anatomy
The cluneal nerves are divided into the superior cluneal nerve (SCN), middle cluneal nerve (MCN), and inferior cluneal nerve (ICN).
The cutaneous nerve supply of the upper back is supplied by the medial branches of the upper thoracic dorsal rami, while the lower back is supplied by the lateral branches of the lower thoracic and upper lumbar dorsal rami. These are called the superior cluneal nerves, and are composed of the lateral cutaneous branches of the dorsal rami of T11-L4 (some authors state T12-L3 or L1-3). There is variability in which of the lateral branches become cutaneous. In embryos and fetuses L1 is always cutaneous, L2 in 90%, L3 in 70%, and L4 in 40%. In adults it is similar except L4 cutaneous branches are uncommon.[2] In 37 dissections, the low back was innervated by the lateral branches of the dorsal rami of T12 and L1 in 60% of cases, T12-L2 in 27% of cases, and T12-L3 in 13% of cases (L1 and L2 receiving an anastomosis from L3).[3]

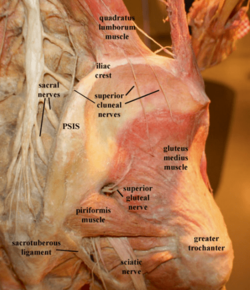
SCN: superior cluneal; MCN: middle cluneal nerve; ICN: inferior cluneal nerve
© Image courtesy of Andrea Trescot, MD[1]
The lateral branches emerge from the dorsolateral border of iliocostalis lumborum, become cutaneous, pierce the thoracolumbar fascia, and descend inferolaterally across the iliac crest to innervate the posterior iliac crest and superomedial gluteus maximus. When crossing the iliac crest they run parallel to one another with those from lower levels lying most medially.[2] These different branches are called the medial branches (mSCN), intermediate or middle branches (iSCN), and lateral branches (lSCN). Do not confuse the middle and medial SCN branches with the middle cluneal nerve (MCN).
The lowest and most medial nerve (mSCN) arises from the deep fascia and crosses the iliac crest approximately 7-8cm from the midline. The mSCN often passes through an osseofibrous tunnel made up of the thoracolumbar fascia and the PSIS.[4] There is significant anatomical variation. In a cadaver study of 109 medial branches, 39% passed through and osseofibrous tunnel, and of these only 5% exhibited obvious entrapment, or 2% of the total dissected branches.[5]
Concept Validity as Source of Chronic Pain
Kuniya and colleagues reported on prevalence data from 834 patients with chronic low back pain in an open access article. This is the best research that I could find in this area but it has multiple significant shortcomings.[6] Their team has published other recent literature on the topic. A recent open access review article on SCN and MCN entrapment was authored by Isu et al.[7]
They classified 14% of patients as suspected superior cluneal nerve entrapment, for which the criteria was
- maximal tender point on the posterior iliac crest approximately 70 mm from the midline and 45 mm from the posterior superior iliac spine where the medial branch of the SCN runs through an osteofibrous tunnel consisting of the thoraco-lumbar fascia and the iliac crest and
- palpation of the maximally tender point reproduced the chief complaint of LBP and/or leg symptoms.
For those who met both criteria they did a nerve block. Unfortunately they did not specify the technique of their nerve block. This is important for us as readers to be sure they were target specific and the anaesthetic didn't spread to other structures such as the ipsilateral sacroiliac ligaments, facet joints, or other branches of the dorsal rami. We would want low volume of anaesthetic injected around the superior cluneal nerve under direct visualisation with ultrasound, and fluoroscopy with contrast to confirm lack of spread. It is highly possible that their cluneal nerve injections were actually trigger point injections. The magnitude and duration of the response reported by the authors is highly consistent with published reports on the effects of trigger point injections.
Of those they did a nerve block in, they reported that 11% of the original 834 had a VAS reduction of 20mm or more (0-100mm scale), and 8% had a VAS reduction of 50% or more. Unfortunately they didn't report on the percentage that had 80% or 100% relief. They described one patient who had 100% relief, but didn't tell us if they were the only one.
The 20mm reduction is a measure of therapeutic effect not of diagnostic validity. It tells us that the injections they did had a clinically meaningful effect in terms of pain reduction. It tells us nothing about the diagnosis. For diagnostic validity you want ideally 100% relief, but 80% could be arguably acceptable. Furthermore, it is very likely that a single block would have a high false positive rate, as that is what we see with single medial branch blocks for facet joint pain, due to the placebo effect. To overcome this we need to see at minimum a second block with a different local anaesthetic that has a different duration of action. The patient should be blinded to which anaesthetic is used. The response should be concordant, i.e. the patient should have a longer response to the longer acting anaesthetic, and a shorter response to the shorter acting one. In an even more ideal world, there would be a third placebo block, with the order all randomised. But because the purported pathology is nerve entrapment, we would need an anatomical not a pharmacological placebo. i.e. the placebo local anaesthetic block would need to be an injection into an adjacent structure like one of the erector spinae muscles. A pharmaceutical block would mean injecting normal saline around the nerve, which would potentially relieve symptoms due to the supposed pathology.
At the moment all we can say is that the potential upper limit for the prevalence of superior cluneal nerve entrapment is 8%, however it is likely to be much lower than that due to the above issues. It is a potentially compelling concept but we need further research with more stringent methodology. We also need to see patients in future prevalence studies having the standard common causes of chronic low back pain ruled out first (internal disc disruption, zygapophysial joint pain, and sacroiliac joint pain where appropriate).
Furthermore we also need to see studies on normal volunteers to see whether noxious injections of hypertonic saline can cause the pain, and whether blocking the superior cluneal nerves can protect against pain from hypertonic saline in these normal subjects. The noxious injections could be done to the thoracolumbar junction structures such as the facet joints.
Pathophysiology
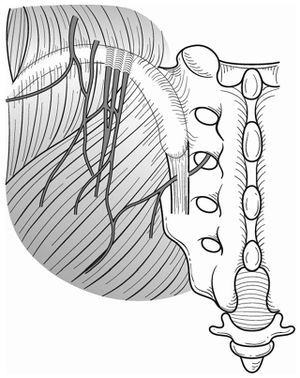
Cluneal nerve irritation may arise due to entrapment in fascia or from nerve root irritation in the epidural space or foramen. Fascial entrapment may be a self-perpetuating process.[9] Pain may occur following spinal fusion, sacroiliac screw placement, sacral ulcer debridement, muscle flap surgeries, iliac bone harvesting, trauma, muscle spasm, spinal canal stenosis, disc herniation, scoliosis, vertebral fractures, and Parkinson's disease. [4]
Superior cluneal nerve (SCN) entrapment is thought to occur where the nerve pierces the thoracolumbar attachment at the posterior iliac crest as it becomes superficial. Typically it has been described based on cadaver studies that the medial branch of the SCN passes through an osteofibrous tunnel and can be spontaneously entrapped there.[4]
However, Kuniya and colleagues found that in their 19 subjects requiring SCN release there were no cases of severe constriction within a bony groove on the iliac crest, but they found at least two SCN branches passing underneath a mixture of superficial thoracolumbar and gluteal fascia at the point of attachment to the iliac crest. They posited that true construction in the osteofibrous tunnel was rare, and that severe symptoms may be due to repetitive friction of the SCN under the fascia. In a further unpublished 26 cases, they had two cases of fascial constriction over the ilium.[6]
The prevalence of vertebral compression fractures is higher in those with SCN entrapment than in those without a SCN disorder (26% vs 12%), most commonly at T12-L3. It is theorised that this causes a sagittal imbalance with increased kyphosis of the spine, thereby irritating the SCN at its origin from unstable facet joints or by stretching the SCN itself.[6] Maigne described thoracolumbar syndrome, whereby thoracolumbar dysfunction causes one of or a combination of unilateral iliac crest pain, lateral hip pain, and groin pain. Knight et al hypothesises that pelvic malrotation and loss of sagittal balance may be an important aetiological factor, even without vertebral fracture and irritant angulation of the cluneal nerve pathway. [9]
Clinical Features
The cardinal symptom is chronic low back pain with or without leg symptoms. Knight and colleagues believe that pelvic malalignment may be a relevant finding with pelvic anterior anteversion, and this may explain why transitional movements can be painful. Cluneal nerve pathology may exist in tandem with other spinal pathology. Symptoms may mimic sciatica.[9] Symptoms may have a neuropathic quality.[8]
In those with a SCN disorder, around 50% have leg symptoms, but only 1% have leg symptoms only. The most commonly aggravating features are walking, rising from sitting, standing, flexion, and extension. Patients often find that pushing above the iliac crest with their hands relieves symptoms. Some patients have claudication like symptoms. The pain can persist for many years and some patients can be severely disabled.[6]
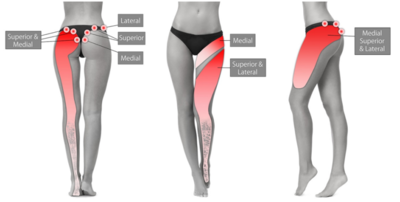
For medial branch SCN pain, there should be maximal tenderness over the PSIS approximately 70mm from the midline and 45mm from the PSIS. coupling rotation to contralateral side and flexion can aggravate symptoms. As can flexion and contralateral rotation. Ipsilateral hip extension may reduce pain during flexion. [6] The medial SCN branches may pass around a small palpable lipoma.[9]
For the lateral nerve group there may be tenderness near the mid-point of the iliac crest, lateral to the tubercle. They tend to be more variable in location and therefore point of irritation.[9]
- Figure: Cluneal nerve pain distribution.[9]
Diagnosis
There is no agreed diagnostic method. The diagnosis can be considered by tenderness over the iliac crest causing provocation of symptoms. Pain relief following local anaesthetic injection can give further weight to the diagnosis.
Kuniya and colleagues used the following diagnostic criteria [6]
- Maximal tenderness over the posterior iliac crest approximately 70 mm from the midline and 45 mm from the PSIS where the medial branch of the SCN runs through an osteofibrous tunnel consisting of the thoraco-lumbar fascia and the iliac crest (even if lesser tenderness exists elsewhere, for example at the thoracolumbar junction).
- Palpation of the maximally tender point reproduced the chief complaint of LBP and/or leg symptoms (Tinel-like sign)
- Temporary relief of symptoms by local anaesthetic injection
In their study, patients were included when they met the first two criteria. A nerve block injection was performed. If relief of symptoms is obtained following injection they suggest that this gives further weight to the diagnosis. In their study, if repeated injections failed to relieve symptoms then they had SCN decompression.
I propose amending their third criteria to requiring positive controlled blocks with at least 80% (ideally 100%) relief and a concordant response to the different agents. The block should be done under ultrasound guidance with a small volume of anaesthetic injected around the superior cluneal nerve - I propose no more than 1.5mL.
This would be more consistent with the diagnostic methodology for the other much more evidence based causes of chronic low back pain.
Mechanical Low Back Pain (97%)
- Internal disc disruption
- Facet joint pain
- Sacroiliac joint pain
- Sacroiliac ligament pain
- Torsion injuries
- Vertebral or sacral insufficiency fractures
- Interspinous tissue injury
- Lamina impaction
- Scoliosis
- Spondylolysis in a sportsperson, but not the general population
- The following are disputed causes of low back pain: degenerative changes, low grade disc infection, non-specific chronic low back pain, transitional vertebrae, congenital fusion, spina bifida occulta, spondylolisthesis, instability, failed back surgery syndrome, back mice, cluneal nerve entrapment, spinal stenosis, myofascial syndrome
Nonmechanical Spine Conditions (1%)
- Neoplasia (Multiple myeloma, metastatic carcinoma, lymphoma, leukaemia, spinal cord tumours, retroperitoneal tumours, primary vertebral tumours)
- Infection (Osteomyelitis, discitis, paraspinous abscess, epidural abscess, shingles)
- Spondyloarthritis
- Scheuermann disease, especially type II
- Diffuse idiopathic skeletal hyperostosis
- Osteitis Condensans Ilii
- Paget disease
Visceral Disease (2%)
- Pelvic organ involvement (Prostatitis, endometriosis, chronic pelvic inflammatory disease)
- Renal involvement (Nephrolithiasis, pyelonephritis, perinephric abscess)
- Aortic aneurysm
- Gastrointestinal involvement (Pancreatitis, cholecystitis, ulcer)
Treatment
Manual and Physical Therapy
Maigne would treat thoracolumbar syndrome with manipulation. Others have used pelvic mobilisation and pelvic realignment. Pelvic malalignment may be well-established and difficult to reverse, and Muscle Balance Therapy or Reformer Pilates could be considered.[9]
Injections
- Main article: Superior Cluneal Nerve Injection
Trigger point injections have been described, normally with plain local anaesthetic, but it can be done with additional steroid or as dextrose prolotherapy. In Kuniya et al's study using the diagnostic criteria above, 47% required a second local anaesthetic injection, and 25% required a third injection. In those who met the first two diagnostic criteria, 62% of patients had a 50% decrease of VAS after the first injection, 67% after the second injection, and 68% after the third injection.[6]
There is very rare risk of erectile dysfunction following injections to the SCN.[6]
An ultrasound guided injection technique is shown here
Radiofrequency neurotomy has also been described [9]
Surgery
Reports have been published on SCN release. In Kuniya's study, approximately 20% of patients proceeded to SCN decompression due to failure of having long term relief from repeated SCN nerve blocks. Predictors of success were pain duration less than 3 years, and consistently having relief for three days or more from SCN blocks.[6] Long term outcomes are satisfactory.[10]
References
- ↑ 1.0 1.1 Trescot, Andrea. Peripheral nerve entrapments : clinical diagnosis and management. Switzerland: Springer, 2016.
- ↑ 2.0 2.1 Bogduk, Nikolai. Clinical and radiological anatomy of the lumbar spine. Edinburgh: Elsevier/Churchill Livingstone, 2012.
- ↑ Maigne et al.. The lateral cutaneous branches of the dorsal rami of the thoraco-lumbar junction. An anatomical study on 37 dissections. Surgical and radiologic anatomy : SRA 1989. 11:289-93. PMID: 2533408. DOI.
- ↑ 4.0 4.1 4.2 Karri et al.. Pain Syndromes Secondary to Cluneal Nerve Entrapment. Current pain and headache reports 2020. 24:61. PMID: 32821979. DOI.
- ↑ Kuniya et al.. Anatomical study of superior cluneal nerve entrapment. Journal of neurosurgery. Spine 2013. 19:76-80. PMID: 23641672. DOI.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 Kuniya et al.. Prospective study of superior cluneal nerve disorder as a potential cause of low back pain and leg symptoms. Journal of orthopaedic surgery and research 2014. 9:139. PMID: 25551470. DOI. Full Text.
- ↑ Isu et al.. Superior and Middle Cluneal Nerve Entrapment as a Cause of Low Back Pain. Neurospine 2018. 15:25-32. PMID: 29656623. DOI. Full Text.
- ↑ 8.0 8.1 Aota. Entrapment of middle cluneal nerves as an unknown cause of low back pain. World journal of orthopedics 2016. 7:167-70. PMID: 27004164. DOI. Full Text.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Martin Knight., et al. “A Radiofrequency Treatment Pathway for Cluneal Nerve Disorders”. EC Orthopaedics 11.3 (2020): 01-19 Full Text
- ↑ Morimoto et al.. Long-term Outcome of Surgical Treatment for Superior Cluneal Nerve Entrapment Neuropathy. Spine 2017. 42:783-788. PMID: 27669049. DOI.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,