Visceral Pain
There are two types of visceral pain: true visceral pain that arises from the viscus or part of a viscus, and pain that arises from the parietal peritoneum or pleura.[1]
Viscero-visceral and viscero-somatic convergence are important clinical concepts. Treatment of one condition may improve symptoms of another when linked through segmental innervation. The resolution of a visceral disease may not resolve the somatic manifestations. Visceral pain manifests some of the same mechanisms seen in somatic pain, but there are crucial differences in central nociceptive processing. Visceral pain also has a strong affective component and can be reinforced by anxiety and depression.[2]
Mechanisms
Visceral pain can be caused by the following mechanisms:[3]
- Spasm of smooth muscle in the hollow viscera
- Contraction of smooth muscle in the gastrointestinal or genitourinary tracts against obstruction
- Sudden, abnormal stretching, distension, or tearing
- Rapid stretching of the capsule of solid viscera
- Rapidly developing ischemia
- Inflammation of the lining of hollow viscera
- Mechanical or chemical stimulation of inflamed mucous membranes
- Traction compression or twisting of the mesentery, organ ligaments, or their blood vessels and necrosis of viscera such as the pancreas or myocardium
- Stimuli that tend not to produce visceral pain, when applied, include cutting, pressure, and burning
Visceral Innervation
The viscera are innervated by one or both of two sets of primary afferent systems that project to different areas of the neuraxis. This innervation supply makes up around 10% of all afferent pathways into the spinal cord.
- Vagus and pelvic splanchnic nerves: The vagus nerve innervates the heart, lungs, and the gastrointestinal tract from the oesophagus to the transverse colon. It projects centrally to the nucleus of the solitary tract. The pelvic splanchnic nerves innervate the remainder of the colon and pelvic organs. They project to the sacral spinal cord. The vagus nerve conveys predominantly physiological information not nociceptive signals but it can modify spinal cord nociceptive processing.
- Thoracolumbar sympathetic trunk splanchnic nerves: These nerves innervate all the thoracic, abdominal, and pelvic viscera. Their afferents terminate in the dorsal gray column between T1 and L2. This system conveys noxious stimuli.
All layers of a viscus are innervated by sensory afferents. These afferents are exclusively small diameter, myelinated A-δ, or unmyelinated C fibres. They have chemosensitivity, thermosensitivity, and/or mechanosensitivity.
The receptors fall into two main different populations, and possibly a third population.
- High-threshold receptors respond to stimuli only within the noxious range. These are found mostly in organs where pain is the main sensation such as the heart and ureter.
- Low-threshold receptors respond to the intensity of a stimulus by increasing their discharge frequency from the innocuous into the noxious range. These are found mostly in the bowel and bladder and provide these viscera with the ability to respond to both innocuous and noxious stimuli such as filling and distension.
- Silent nociceptive receptors are sensitised by ischaemia and inflammation
Both central and peripheral sensitisation can occur, just like the somatic nociceptive system. Functional visceral disturbance can persist even after the initial insult has resolved. But there are some differences in the pathophysiology of central sensitisation.
While some receptors are silent and recruited by tissue injury and inflammation, all can be sensitised and drive central sensitisation that can persist even after the resolution of an initial insult. Damage and inflammation also affects normal motility and secretion, producing changes to the nociceptor environment. Ascending pathways include the spinothalamic tract and dorsal column. Ascending tracts synapse at the thalamus, limbic centres, and the somatosensory cortex. There is no somatotopic representation in the cortex. Pain is represented in the secondary somatosensory cortex. Visceral pain elicits nausea and hypotension, the opposite to somatic pain.
Peripheral sensitisation can occur, and there are some similarities with the somatic system. Low- and high-threshold receptors relay acute pain. silent receptors are sensitised. Inflammation lowers receptor firing thresholds, facilitating central transmission. In the gut there is increased activity of a subpopulation of sodium channels which mediates this increased activity. Compared to the somatic system, the visceral system has a higher amount of substance P, CGRP, somatostatin, and VIP.
Central sensitisation can occur but the mechanism is thought to be different to the somatic system. NMDA receptors are thought to play a role in the transmission of both innocuous and acute noxious colonic stimuli, as NMDA receptor antagonists inhibit transmission. Meanwhile in the somatic system NMDA receptors do not play these roles, but rather contribute to the development of central sensitisation. In irritable bowel syndrome it may be the case that there is increased NMDA receptor activity without noxious stimuli. NK-1 receptors signal neurogenic inflammation, but not non-neurogenic inflammation.[4]
Clinical Characteristics
Visceral pain is initially described as a vague, central sensation felt anterior or posterior, accompanied by various autonomic and neurovegetative symptoms such as malaise, nausea, sweating, palour, anxiety, changes in blood pressure and heart rate, and sense of impending doom (‘true visceral pain’). Eventually this subsides, and is followed by a variety of symptoms and signs perceived in somatic areas receiving the same innervation. Skin, subcutis and muscle are all involved. Initially, the symptoms of pain rather than pain itself is felt. As time progresses, signs start to develop. The muscle layer is earliest and most commonly involved layer. However, the skin and subcutaneous layers are also commonly involved with hyperalgesia, and sudomotor signs. These symptoms and signs can persist even when the visceral insult has resolved. These symptoms and signs may also, in turn, influence visceral symptoms.
| Symptoms & Signs | Neurobiology |
|---|---|
| May not be evoked from all viscera | Not all viscera are innervated by sensory receptors or because the stimulus is inappropriate. |
| Not always linked to injury | Functional properties of visceral sensory afferents |
| Referred to the body wall | Viscerosomatic convergence in central pain pathways |
| Diffuse and poorly localised | Few sensory visceral afferents. Extensive divergence in central nervous system |
| Often accompanied by accentuated motor and autonomic reflexes | Mainly a warning system, with substantial capacity for amplification |
Pain is not evoked from all viscera
It is generally evoked from the liver, kidney, lung parenchyma, and most solid viscera. These features are due to the functional properties of the peripheral receptors of the nerves that innervate certain visceral organs and to the fact that many viscera are innervated by receptors that do not evoke conscious perception and, thus, are not sensory receptors in the strict sense
Pain is not linked to injury
Cutting the intestine causes no pain. But stretching it does (without injury). Pain can be produced by ischaemia, inflammation of the lining of the hollow viscus, stimulation (chemical or mechanical) of inflamed mucous membranes, traction compression or twisting of the mesentery, organ ligaments or blood vessels, or necrosis of the viscera such as the pancreas or myocardium.
Visceral pain is referred to the body wall
- See also: Referred Pain
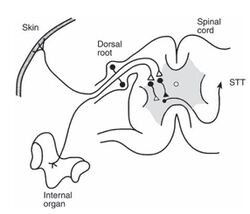
Viscero-somatic convergance and referred pain, muscle spasm, and hyperalgesia. All viscera are innervated by thoracolumbar outflow of sympathetic nervous system T1 - L2 via cardiac and splanchnic nerves, with cell bodies in the dorsal root ganglion. During development, viscera change position, drawing their innervation with them. This leads to a mismatching of intra abdominal position and segmental innervation, and enables understanding of pain referral patterns. Visceral sensations are mediated through convergent signals via somatosensory pathways.
Visceral afferents and cutaneous nociceptors converge on similar neurons at some point in the sensory pathways probably at a spinal level, but could occur at thalamic and cortical levels. The resulting impulses may be then misinterpreted at a spinal level as originating in the skin or other somatic structure. Most second order neurons receiving visceral input in laminae I and V, some in ventral horn. Fewest in the superficial dorsal horn. Most in the deep dorsal and ventral horn. Here they have diffuse and bilateral visceral and somatic input, are subject to descending excitatory and inhibitory control. Visceral afferents comprise fewer than 10% of all spinal afferent input. Therefore, visceral sensations can be mediated only through convergent signals via somatosensory pathways.
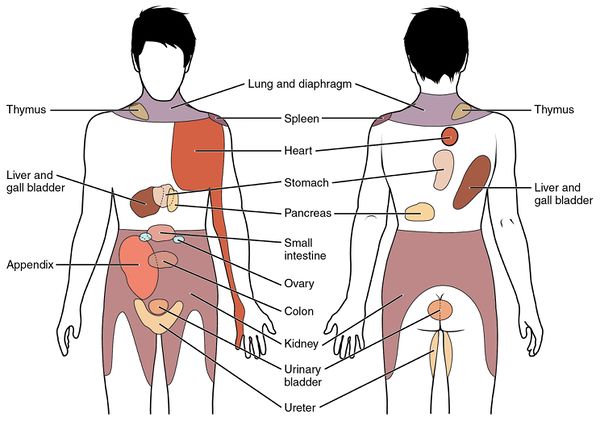
Some of the classic examples of viscero-somatic referred pain are: diaphragm to C4; heart to C8-T8; bladder to T1-T10; stomach to T6-T9; intestine to T7-T10; testes, prostate, and uterus to T10-T12, kidneys to T11-L1, and rectum to S2-S4.[6]
| Structure | Segmental Innervation | Possible Pain Locations |
|---|---|---|
| Above the Diaphragm | ||
| Oesophagus | T4-6 | Substernal and upper abdominal |
| Heart | T1-5 | Cervical anterior, jaw, teeth, upper thoracic, epigastric, left upper arm, right shoulder and upper arm |
| Lungs and Bronchi | T1-5/6 | thoracic (ipsi), chest wall, cervical (diaphragm involved) |
| Diaphragm (central portion) | C3-5 | Cervical Spine |
| Below the Diaphragm | ||
| Stomach | T6-T10 | Upper abdominal, middle and lower thoracic spine |
| Gallbladder | T7-T9 | RUQ, central and right middle and lower thoracic, right scapula |
| Liver | T7-T9 | Right mid and lower thoracic, right cervical |
| Common Bile Duct | T8-10 | Upper abdominal, mid thoracic |
| Pancreas | T10 | Upper abdominal, lower thoracic, upper lumbar |
| Small Intestine | T7-T10 | Middle thoracic spine |
| Kidney | T10-L1 | TL junction (ipsi), mid lumbar, lower and upper abdominal |
| Ureter | T11-L2, S2-S4 | Groin, med/prox thigh, upper abdominal, suprapubic, TL, iliac crest |
| Large Intestine | T11-L1 | Lower abdominal, mid lumbar, buttock |
| Sigmoid Colon | T11-12 | Upper sacral, suprapubic, LLQ |
| Uterus (including uterine ligament) | T1-L1, S2-S4 | LS junction, sacral, TL |
| Ovaries | T10-T11 | Low abdominal, sacral |
| Testes | T10-T11 | Lower abdominal, sacral |
| Bladder | T11-L2, S2-S4 | Sacral apex, suprapubic, TL |
| Prostate | T11-L1, S2-S4 | Sacral, perineal, testes, thoracolumbar |
| TL = thoracolumbar. LS = lumbosacral. From Boissonnault W, Bass C. Pathological origins of trunk and neck pain: parts I, II, III. J Orthop Sports Phys Ther 1990;12:191–221. This in turn isn't referenced properly. I think lung was wrong, which I've fixed. | ||
Visceral pain can lead to somatosensory disturbances. in 1893 the neurologist Sir Henry Head published his findings of areas of cutaneous sensitivity and maximal tenderness in cases of visceral pathology (T1-S4). These have been called "Head Zones" and are similar to dermatome charts.[7]
In recent one study of 100 patients with appendicitis, 39 had abdominal wall somatosensory disturbances in the right lower quadrant such as discriminative sensibility changes with a swab, vital sensibility with alcohol gauze, skin fold squeezing sensitivity, and/or positive Carnett's test. [8]
A related concept are the Chapman points in Osteopathy.[9]
It is often not possible to differentiate visceral from musculoskeletal pathology, hence visceral pathology should be excluded first in the appropriate clinical settings. Treatment of the musculoskeletal manifestations without addressing the visceral cause, may result in masking the visceral cause. The somatosensory disturbances can be diagnostically useful however in determining the segmental level of the visceral insult.
Somatosensory disturbances may persist after treatment of the underlying visceral disorder.
Visceral pain is diffuse and poorly localised
Due to low density of sensory innervation, and extensive divergence of visceral input within CNS. Low proportion of visceral afferent fibres compared to somatic origin. A few visceral afferents can activate many neurons in the spinal cord. Viscerovisceral convergance contributes to difficulty in pinpointing source of pain. There is also poor representation in the primary somatosensory cortex.
- Viscerovisceral Hyperalgesia:
This can occur in patients presenting with more than one painful visceral condition at a time. Occurs if affected organs have share segmental innervation. These include the heart and gallbladder (T5), the uterus and upper urinary tract (T10-L1), the uterus and colon (T10-L1), and the bladder and sigmoid colon/rectum (L1-L2). Enhancement of typical symptoms from all affected viscera, as well as referred phenomenon. Effective treatment of one visceral disease may relieve symptoms from the other disease. Treatment of muscle hyperalgesia may relieve pain of one or both.
- VSR & VVR clinical observations
Effectively treating IHD can ease severity of pain from GB disease and vice versa. Treating dysmennorhoea has been shown to reduce urinary tract pain, pain from IBS, and muscle hyperalgesia. VVH can occur when one condition is ‘silent’ treatment of endometriosis discovered at laparoscopy has reduced urinary symptoms. Effectively treating muscle tenderness can ease pain of dysmennorhoea , IBS and urinary tract.
Visceral pain is associated with intense motor and autonomic reactions
There is the potential for afferent branching to synapse with autonomic efferent fibres. There are both spinal and supraspinal mechanisms.
Naming issues
Many authors fail to appreciate the potential significance of VSC, VSR, VVR. Abdominal wall cutaneous nerve entrapment syndrome, Myofascial pain, Regional pain syndrome, Non specific abdominal wall pain.
Clinical Implications
Neurobiology can help us understand at least some of the basis for MSK pain that may be linked to visceral dysfunction. Numerus studies have demonstrated that manifestations of viscero-somatic convergance can persist after resolution of visceral disease (pain, muscle spasm, hyperalgesia). Hyperalgesia can extend beyond expected segment (diffuse muscle hyperalgesia). Local treatment of muscle hyperalgesia may reduce visceral symptoms. History needs to include assessing current and past visceral symptoms, and timeframe of symptom development. Understanding the VSR can provide insight into the genesis of peripheral symptoms and signs. Awareness of VSR and VVR reminds us that MSK symptomatology should not be treated in isolation, and may involve other disciplines.
VVR can result in increased pain in both viscera, AND muscle hyperalgesia. Effective treatment of one visceral disease can alleviate other spontaneous pain and referred muscle hyperalgesia. VVH can take place when one of the two visceral conditions is latent, or silent.
References
Thank you to Dr Bell for his presentation on the topic.
- ↑ Lewis, T. (1942). Pain. New York: Macmillan.
- ↑ Sikandar & Dickenson. Visceral pain: the ins and outs, the ups and downs. Current opinion in supportive and palliative care 2012. 6:17-26. PMID: 22246042. DOI. Full Text.
- ↑ Procacci, P., Maresca, M., & Cersosimo, R. M. (1991). Visceral pain: Pathophysiology and clinical aspects. Advances in Experimental Medicine and Biology, 298, 175–181.
- ↑ Borowczyk J. (2013) Visceral Pain and Nociception. In: Schmidt R., Willis W. (eds) Encyclopedia of Pain. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-29805-2_4801
- ↑ Cervero & Laird. Visceral pain. Lancet (London, England) 1999. 353:2145-8. PMID: 10382712. DOI.
- ↑ Andre Parent. Carpenter's Human Neuroanatomy. 1996
- ↑ Henry Head. On disturbances of sensation with especial reference to the pain of visceral disease. Brain, Volume 16, Issue 1-2, 1893, Pages 1-133. https://doi.org/10.1093/brain/16.1-2.1
- ↑ Roumen RMH, Vening W, Wouda R, Scheltinga MM. Acute Appendicitis, Somatosensory Disturbances ("Head Zones"), and the Differential Diagnosis of Anterior Cutaneous Nerve Entrapment Syndrome (ACNES). J Gastrointest Surg. 2017 Jun;21(6):1055-1061. doi: 10.1007/s11605-017-3417-y. Epub 2017 Apr 14. PMID: 28411350.
- ↑ Bath M, Nguyen A, Bordoni B. Physiology, Chapman’s Points. [Updated 2021 May 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558953/