Nonsteroidal Anti-Inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are members of a drug class that inhibit the activity of cyclooxygenase (COX-1 or COX-2) enzymes. This confers them analgesic, antipyretic, and anti-inflammatory effects, as well as inhibition of platelet aggregation. Side effects depend on the specific drug but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease. This article should be read in conjunction with Prostaglandins and Cyclooxygenase Enzymes.
Pharmacology of NSAIDs
All NSAIDs, except aspirin (see below), act as competitive reversible inhibitors of COX, thus reducing the formation of various prostaglandins.
The traditional NSAIDs (ibuprofen, diclofenac, etc) operate on locations of the COX enzyme that are identical in both isoenzymes. This results in blocking of both the inflammatory effects of COX2 and the normal physiological effects of COX1. This results in potential side effects particularly on the kidney and stomach.
The selective COX2-inhibitors (celecoxib, meloxicam, etc) bind to a site on COX2 that isn't present or accessible on COX1. which reduces the side effects related to COX1 inhibition. There is overlap in the selectivity of some traditional NSAIDs with selective COX-2 inhibitors like diclofenac and celecoxib which have similar COX-1/COX-2 IC50 ratios (the measure of selectivity of COX isoforms).[1]
The except to the rule of competitive reversible inhibition of COX is aspirin. Aspirin irreversibly acetylates a key amino acid (serine 529) on the active site of COX with a covalent bond, leading to permanent enzyme inhibition by interfering with the binding site of arachidonic acid at the active site on COX. This covalent binding means that the antagonistic effects only cease when the receptor or membrane are replaced.
Another slight exception is indomethacin, a old NSAID that is no longer available in New Zealand. It evolves from non-competitive binding to permanent binding.
COX inhibitors antagonise central hyperalgesia in the dorsal horn through modulating the glutamatergic signalling from nociceptive C fibres to secondary neurons. COX-2 but not COX-1 inhibition suppresses inflammation-induced prostaglandin level rise the CSF.
Paracetamol also deserves special discussion. It was traditionally stated that it acted centrally and was a weak inhibitor of COX-1 and COX-2. It actually acts on both peripheral and central COX enzymes, is a weak anti-inflammatory agent, and has preferential COX-2 inhibition. It acts on COX as a reducing agent within the peroxidase site.
Adverse Effects and Prescribing Considerations
Many of the side effects of NSAIDs are due to COX-1 suppression, such as gastrointestinal ulceration and bleeding, and platelet dysfunction. Selective COX-2 inhibitors were developed with the idea of avoiding the unwanted side effects that are elicited by COX-1 inhibition.
Gastrointestinal Effects
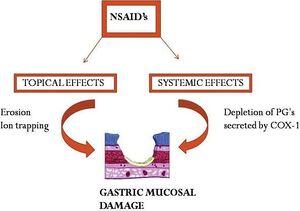
The gastrointestinal side effects are due to both direct irritation and through reduction of prostaglandin synthesis.
Normally, the "good" prostanoids stimulate the secretion of mucus and bicarbonate, increase blood flow, and promote epithelial proliferation. By inhibiting prostaglandin synthesis, the gastric environment is more susceptible to topical erosion by both exogenous and endogenous factors.
The acidic properties of most NSAIDs initiate mucosal damage. The drug molecules remain in a non-ionised lipophilic form in the acid environment of the stomach. They enter into surface epithelial cells where they dissociate, and trap hydrogen ions, inducing lesions. This is called "ion trapping". They also reduce the hydrophobicity of gastric mucus, allowing HCL and pepsin to damage the epithelial surface.
Compounding the above, is that a reduction of thromboxane results in a favourable bleeding environment.
The risk of peptic ulcers in high risk patients can be significantly reduced by the co-administration of a proton-pump inhibitor. However this combination does not protect against damage to the lower gastrointestinal tract. Using COX-2 inhibitors rather than traditional NSAIDs also reduces the risk.[2]
The co-administration of SSRIs also increases the risk of GI bleeding. Alcohol consumption also increases the risk of GI bleeding. The effect of alcohol abuse is particularly potent, with an OR of 10.2 when in those who also take NSAIDs.[3]
Some practical guidelines for prescribing with regards to gastrointestinal risk:
- General: Use the lowest dose for the shortest time. Avoid prescribing concurrently with anticoagulants, corticosteroids, and spironolactone. In patients with a high pre-test probability for helicobacter pylori infection, consider testing and eradication.
- NSAIDs with very high GI toxicity are not generally available in New Zealand (piroxicam, ketoprofen, ketorolac).
- Low gastrointestinal risk: use non-selective NSAIDs
- Moderate to high gastrointestinal risk: use selective COX-2 inhibitors or non-selective NSAIDs plus PPIs.
Cardiovascular Effects
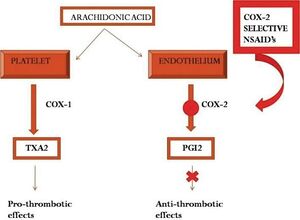
The long term use of both traditional NSAIDs and COX-2 inhibitors confers cardiovascular risks. The mechanism for this relates to the previously unrecognised role of COX-2 expression in basal conditions, and the blockade of COX-2 dependent prostaglandins such as prostacyclin.
Prostacyclin is an inhibitor of platelet aggregation and also has vasodilatory properties, and is produced by endothelial cells. Prostacyclin is reduced by over 60% by both traditional NSAIDs and COX-2 inhibitors. Theoretically, the increased risk of vascular events would be mitigated by reduced production of thromboxane A2 by platelets. Thromboxane is a prostanoid that promotes platelet aggregation. However, COX-2 inhibitors, having by definition no affinity for COX-1, do not reduce the production of thromboxane by platelets. These effects on thromboxane and prostacyclin may create a prothrombotic state.
It was therefore previously thought that COX-1 inhibition was protective due to inhibition of platelet aggregation through the thromboxane effect. But this effect only occurs with suppression of >95% of COX-1 activity. This level of suppression is only seen with low-dose aspirin and in some people with naproxen dosed at 500mg twice daily. This is why traditional NSAIDs (ibuprofen, diclofenac), except naproxen, are also associated with a cardiovascular risk. In fact, the rate of serious vascular events is similar to COX-2 inhibitors and any non-naproxen NSAID.[2]
Rofecoxib (Vioxx) and valdecoxib (Bextra) were withdrawn from the market due to the increased number of severe thromboembolic events with short term treatment. This has resulted in the change in labelling of all COX inhibitors to encourage taking them at the lowest effective dose for the shortest possible duration.
Some practical guidelines for prescribing with regards to CVD risk:[2]
- Low CVD risk: Prescribed either a non-selective NSAID or a selective COX-2 inhibitor. The choice should be dependent on the gastrointestinal risk.
- Intermediate CVD risk: choose from ibuprofen or naproxen plus a proton pump inhibitor
- High CVD risk: Naproxen plus PPI. Selective COX-2 inhibitors should be avoided.
- In those who take aspirin: Take aspirin 2 hours before naproxen or 8 hours before ibuprofen to avoid reducing clinical efficacy of aspirin.
Renal Effects
Prostaglandins are important mediators of kidney function. They help to maintain blood volume and electrolyte balance, and also control the release of renin and contribute to renal vasodilation. COX-1 regulates renal haemodynamics and glomerular filtration. COX-2 mediates salt and water excretion. Therefore all NSAIDs can alter renal function.
The effect on salt and water retention is due to the loss of prostaglandin induced action on ADH. This can increase blood pressure. Naproxen can increase the mean arterial pressure by 3-4mmHg.
Nephropathy is a rare side effect. It is more likely in high doses, and in patients with CHF, CKD, hypovolaemia, and disorders of the RAAS system. Interstitial nephritis, nephrotic syndrome, and papillary necrosis are all underlying pathophysiological mechanisms. NSAIDs should be avoided in those with chronic kidney disease.
Indomethacin has the highest risk for nephropathy. Intermediate risk drugs are ibuprofen, naproxen, and diclofenac. The risk of selective COX-2 drugs on renal function is not clear.[4]
Due to risk of acute kidney injury, NSAIDs should be prescribed with caution in those who take ACE inhibitors. NSAIDs should be avoided completely in those that take both ACE inhibitors and thiazide diuretics - the so called "triple whammy."
Hepatic Effects
Hepatotoxicity is a very rare effect, and can manifest and cholestatic, hepatocellular, or mixed injury. The risk is increased substantially in those with rheumatic diseases with a RR of 10.9.
Hypersensitivity
NSAID hypersensitivity includes bronchial asthma, rhinosinusitis, anaphylaxis, urticaria, and a variety of late cutaneous and organ-specific reactions.
COX-1 inhibition is the mechanism for aspirin hypersensitivity and unrelated NSAIDs in some patients with asthma and in some patients with chronic urticaria-angioedema. In these cases, COX-1 inhibition results in activation of the lipoxygenase pathway which induces bronchospasm and nasal obstruction. Arachidonic acid is converted to leukotrienes via the lipoxygenase pathway.
In accordance with the role of COX-1 in aspirin-induced asthma, selective COX-2 inhibitors have reduced risk on re-exposure. Importantly, many traditional NSAIDs have a very high risk of hypersensitivity in aspirin hypersensitive patients (table 2). [5]
- NSAIDs cross-reacting in majority of hypersensitive patients (60-100%):
- Ibuprofen, naproxen, diclofenac, mefenamic acid
- NSAIDs cross-reacting in minority of hypersensitive patients (2-10%):
- Rhinitis/asthma type: paracetamol below 1000mg, meloxicam
- Urticaria/angioedema type: paracetamol, meloxicam, celecoxib
- NSAIDs well tolerated by all hypersensitive patients (except for isolated cases):
- Rhinitis/asthma type: celecoxib
- Urticaria/angioaedema: etoricoxib
Different NSAIDs
Nomenclature
The naming scheme of NSAIDs is related to the base molecule from which they are derived.
- pyrroleACetic - diclofenAC, ketorolAC
- PROpionic - ibuPROfen, naPROxen
- sALicylates - acetylesALicylic acid
- OXycams - pirOXicam
- indoleACetic - indomethACin
Overall Comparisons
| Drug | Chemical Subclass | pKa | Plasma
protein binding |
Oral bioavailability | t-max† | Half life | Single dose
(maximal daily dose) for adults |
COX selectivity | Side Effects (SEs) |
|---|---|---|---|---|---|---|---|---|---|
| Low potency / short elimination half life compounds | |||||||||
| Aspirin |
Salicylates | 3.5 | 50-70% | ~50%, dose dependent | ~15 min | ~15 min** | 0.05-1g (~6g)‡ | COX-1 = COX-2 | Long lasting platelet effect. GIT SEs. Reye's syndrome in children. |
| Ibuprofen |
2-Arylpropionic acids | 4.4 | 99% | 100% | 0.5-2h | 2h | 200-800mg (2.4g) | COX-1 > COX-2 | High doses has increased GIT SEs. CVD RR 1.51[6] |
| Mefenamic acid |
Anthranilic acids | 4.2 | 90% | 70% | 2-4h | 1-2h | 250-500mg (1.5g) | ||
| High potency / short elimination half life compounds | |||||||||
| Diclofenac |
Aryl-/heteroarylacetic acids | 3.9 | 99.7% | ~50%, dose dependent,
high first pass metabolism |
1-12h, variable | 1-2h* | 25-75mg (150mg) | COX-2 ≫ COX-1 | Low rate of GIT SEs, slightly higher liver toxicity, CVD RR 1.63[6] |
| Intermediate potency / intermediate elimination half life compounds | |||||||||
| Naproxen |
2-Arylpropionic acids | 4.2 | 99% | 90-100% | 2-4h | 12-15h | 250-500mg (1.25g) | COX-1 > COX-2 | More ulcer bleeds than ibuprofen. CVR RR 0.92[6] |
| High potency / long elimination half life compounds | |||||||||
| Tenoxicam |
Oxicams | 5.3 | 99% | ~100% | 0.5-2h | 25-175h¤ | 20-40mg; initial: 40mg | ?COX-2 ~ COX-1 | Oxicams have a somewhat higher incidence of GIT and renal effects. |
| Meloxicam |
Oxicams | 4.08 | 99.5% | 89% | 7-8h | 20h¤ | 7.5-15mg | COX-2 > COX-1 | |
| Selective COX-2 inhibitors | |||||||||
| Celecoxib |
Sulfonamides | 11.1 | 97% | 20 - 60% | 2-4h | 6-12h | 100-200mg (400mg) | COX-2 ≫ COX-1 | Less GIT SEs∞. Increased CVD SEs. |
| Etoricoxib |
Methylsulfons | 4.96 | 92% | ~ 100% | 1h | 20-26h | 120mg | COX-2 ⋙ COX-1 | Increased CVD Ses |
| Paracetamol | |||||||||
| Paracetamol |
Aniline derivatives | 5-50% | 70 - 100% | 70 - 100%, dose dependent | 0.5-1.5h | 1.5-2.5h | 0.5-1g (4g) | COX-2 > COX-1 | Less GIT SEs. Unclear CVD SEs. Dose dependent hepatotoxicity. |
|
† Time to reach maximum plasma concentration Modified from Pharmacology of Cyclooxygenase Inhibitors. Encyclopedia of Pain 2013.[7] | |||||||||
Risk Comparisons
| NSAID daily dose | GI adverse events | Major Vascular Events | Heart failure |
|---|---|---|---|
| Celecoxib, etoricoxib | 1.81 (1.17 to 2.81) | 1.37 (1.14 to 1.66) | 2.28 (1.62 to 3.20) |
| Diclofenac 150mg | 1.89 (1.16 to 3.09) | 1.14 (1.12 to 1.78) | 1.85 (1.17 to 2.94) |
| Ibuprofen 2400mg | 3.97 (2.22 to 7.10) | 1.44 (0.89 to 2.33) * | 2.49 (1.19 to 5.20) |
| Naproxen 1000mg | 4.22 (2.71 to 6.56) | 0.93 (0.69 to 1.27) | 1.87 (1.10 to 3.16) |
| *Ibuprofen did however increase coronary events risk with a RR of 2.2 (1.1 to 4.48).
Also note, daily dose of ibuprofen was high at 2400mg. | |||
Ibuprofen (≤1200 mg daily) and naproxen have the lowest cardiovascular risk, but have an increased gastrointestinal risk. Moderate doses of celecoxib may be non-inferior to naproxen and ibuprofen for cardiovascular safety.
Resources
See Also
References
- ↑ Hunter TS, Robison C, Gerbino PP. Emerging evidence in NSAID pharmacology: important considerations for product selection. Am J Manag Care. 2015 Apr;21(7 Suppl):S139-47. PMID: 26168321.
- ↑ 2.0 2.1 2.2 2.3 2.4 Oliviu Vostinaru (August 23rd 2017). Adverse Effects and Drug Interactions of the Non‐Steroidal Anti‐Inflammatory Drugs, Nonsteroidal Anti-Inflammatory Drugs, Ali Gamal Ahmed Al-kaf, IntechOpen, DOI: 10.5772/intechopen.68198. Available from: https://www.intechopen.com/chapters/54761
- ↑ Neutel CI, Appel WC. The effect of alcohol abuse on the risk of NSAID-related gastrointestinal events. Ann Epidemiol. 2000 May;10(4):246-50. doi: 10.1016/s1047-2797(00)00040-5. PMID: 10854958.
- ↑ Zarghi, Afshin, and Sara Arfaei. “Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships.” Iranian journal of pharmaceutical research : IJPR vol. 10,4 (2011): 655-83. cyclooxygenases (COX-1 and COX-2).
- ↑ Kowalski ML, Makowska JS, Blanca M, Bavbek S, Bochenek G, Bousquet J, Bousquet P, Celik G, Demoly P, Gomes ER, Niżankowska-Mogilnicka E, Romano A, Sanchez-Borges M, Sanz M, Torres MJ, De Weck A, Szczeklik A, Brockow K. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy. 2011 Jul;66(7):818-29. doi: 10.1111/j.1398-9995.2011.02557.x. Epub 2011 Feb 14. PMID: 21631520.
- ↑ 6.0 6.1 6.2 Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun 3;332(7553):1302-8. doi: 10.1136/bmj.332.7553.1302. PMID: 16740558; PMCID: PMC1473048.
- ↑ Hinz et al. Pharmacology of Cyclooxygenase Inhibitors. Encyclopedia of Pain 2013. Springer, Berlin, Heidelberg.
- ↑ Bhala N, Emberson J, Merhi A, etal Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769-79. doi: 10.1016/S0140-6736(13)60900-9 pmid: 23726390
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,


