EBQ:SPACE Trial: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{#pmid:29509867}} | {{#pmid:29509867}} | ||
==Clinical Question== | ==Clinical Question== | ||
To compare opioid vs nonopioid medications over 12 months on pain-related function, pain intensity, and adverse effects. | To compare opioid vs nonopioid medications over 12 months on pain-related function, pain intensity, and adverse effects in patients with moderate to severe chronic back or osteoarthritic hip/knee pain. | ||
==Bottom Line== | ==Bottom Line== | ||
In patients with severe back or hip/knee pain that weren't currently receiving opioid treatment, there was no difference between opioid vs. non-opioid treatment over 12 months with an escalating treatment-to-target approach. There may be more adverse events with opioid therapy. | |||
==Study Design== | ==Study Design== | ||
Pragmatic, | * Pragmatic, single-centre, open label, randomized trial | ||
* N=240 | |||
** Opioid (n=120) | |||
** Non-opioid (n=120) | |||
* Setting: 62 American primary care clinicians affiliated with the Veterans Affairs | |||
* Enrolment: June 2013 to December 2015 | |||
* Mean follow-up: 12 months | |||
* Analysis: Intention-to-treat, masked outcome assessment. | |||
* Primary Outcome: Improvement in pain-related function assessed with the Brief Pain Inventory (BPI) | |||
==Population Studied== | ==Population Studied== | ||
| Line 25: | Line 28: | ||
====Adverse events==== | ====Adverse events==== | ||
==Discussion== | ==Discussion== | ||
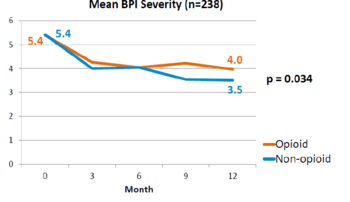
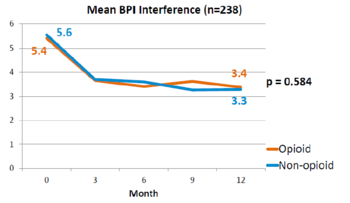
This is a landmark study published in 2018 by Krebs et al - the SPACE study. It was a pragmatic randomised controlled trial comparing opioid versus non opioid analgesics for 12 months in primary care. Participants were 240 VA patients with moderate to severe chronic back pain or knee/hip OA, and not on opioids. The mean pain intensity initially was 5.4 in both arms. Pain scores at 1 year was worse in the opioid arm (4.0) than non opioid (3.5) (P=0.034). There was no difference in pain interference, and adverse effects were worse in opioid group (P=0.03). | |||
<gallery mode="nolines" widths=350px heights=250px> | |||
File:SPACE BPI 2018.png|Brief Pain Inventory (BPI) over 12 months | |||
File:SPACE BPI interference 2018.png|Pain Interference over 12 months | |||
</gallery> | |||
===Criticism=== | ===Criticism=== | ||
*Unblinded | |||
*Patient self-reporting is an area of bias | |||
*The patients were veterans, and so this might limit the external validity | |||
*Patients using opioids already were excluded | |||
*The reported adverse events may not represent the highly concerning ones. | |||
==Funding== | ==Funding== | ||
==See Also== | ==See Also== | ||
Revision as of 19:03, 26 April 2021
Krebs et al.. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA 2018. 319:872-882. PMID: 29509867. DOI. Full Text.
Clinical Question
To compare opioid vs nonopioid medications over 12 months on pain-related function, pain intensity, and adverse effects in patients with moderate to severe chronic back or osteoarthritic hip/knee pain.
Bottom Line
In patients with severe back or hip/knee pain that weren't currently receiving opioid treatment, there was no difference between opioid vs. non-opioid treatment over 12 months with an escalating treatment-to-target approach. There may be more adverse events with opioid therapy.
Study Design
- Pragmatic, single-centre, open label, randomized trial
- N=240
- Opioid (n=120)
- Non-opioid (n=120)
- Setting: 62 American primary care clinicians affiliated with the Veterans Affairs
- Enrolment: June 2013 to December 2015
- Mean follow-up: 12 months
- Analysis: Intention-to-treat, masked outcome assessment.
- Primary Outcome: Improvement in pain-related function assessed with the Brief Pain Inventory (BPI)
Population Studied
Inclusion Criteria
Eligible patients had chronic back pain or hip or knee osteoarthritis pain that was moderate to severe despite analgesic use. Chronic pain was defined as pain nearly every day for 6 months or more. Moderate or greater severity was defined by a score of 5 or more on the 3-item pain intensity, interference with enjoyment of life, and interference with general activity (PEG) scale (range, 0-10).
Exclusion Criteria
Patients on long-term opioid therapy were excluded. Other reasons for exclusion included contraindications to all drug classes in either group, including class-level opioid contraindications (eg, active substance use disorder), and conditions that could interfere with outcome assessment (eg, life expectancy <12 months). Patients with severe depression or posttraumatic stress disorder symptoms were not excluded because these patients often receive opioids in practice.
Baseline Characteristics
Interventions
Outcomes/Results
Primary Outcomes
Secondary Outcomes
Adverse events
Discussion
This is a landmark study published in 2018 by Krebs et al - the SPACE study. It was a pragmatic randomised controlled trial comparing opioid versus non opioid analgesics for 12 months in primary care. Participants were 240 VA patients with moderate to severe chronic back pain or knee/hip OA, and not on opioids. The mean pain intensity initially was 5.4 in both arms. Pain scores at 1 year was worse in the opioid arm (4.0) than non opioid (3.5) (P=0.034). There was no difference in pain interference, and adverse effects were worse in opioid group (P=0.03).
Criticism
- Unblinded
- Patient self-reporting is an area of bias
- The patients were veterans, and so this might limit the external validity
- Patients using opioids already were excluded
- The reported adverse events may not represent the highly concerning ones.