EBQ:SPACE Trial: Difference between revisions
No edit summary |
No edit summary |
||
| Line 18: | Line 18: | ||
==Population Studied== | ==Population Studied== | ||
===Inclusion Criteria=== | ===Inclusion Criteria=== | ||
*Back pain or knee or hip pain associated with osteoarthritis | |||
*Pain occurring almost every day for ≥6 months despite non-opioid analgesic use | |||
*Brief Pain Inventory (BPI) scale average pain ≥5 and BPI interference score ≥5 | |||
===Exclusion Criteria=== | ===Exclusion Criteria=== | ||
*Severe mental illnesses | |||
*Moderate severe cognitive function | |||
*Planned surgery to address pain in the next year | |||
*Long term opioid treatment | |||
*Contraindications to study medications | |||
*Life expectancy <12 months | |||
===Baseline Characteristics=== | ===Baseline Characteristics=== | ||
*Demographics: Mean age 57 years, 13% female, 88% white, 6% black, 6% other ethnicity, higher education 24% | |||
*Employment: Employed 42%, self-employed 6%, retired 36% | |||
*Pain eligibility: Back pain 65%, Hip/knee osteoarthritis pain 35% | |||
*Smoker 21%, hazardous alcohol use (AUDIT Score ≥8) 3%, illicit drug use in prior year 7% | |||
*Mental Health: Moderate depression 23%, Moderate anxiety 9%, PTSD 21% | |||
==Interventions== | ==Interventions== | ||
Participants were randomized to a group in and received stepwise approach to management of pain: | |||
*Opioid Group - Titrated to maximum of 100 morphine-equivalent mg: | |||
*#Morphine IR, hydrocodone/acetaminophen, oxycodone IR | |||
*#Morphine sustained release, oxycodone sustained release | |||
*#Transdermal fentanyl | |||
*Non-opioid | |||
*#Acetaminophen and NSAIDs | |||
*#Adjuvant oral medications (nortriptyline, amitriptyline, gabapentin), topical analgesics (capsaicin, lidocaine) | |||
*#Drugs requiring pre-authorization in VA system (pregabalin, duloxetine, tramadol) | |||
==Outcomes/Results== | ==Outcomes/Results== | ||
===Primary Outcomes=== | ===Primary Outcomes=== | ||
| Line 40: | Line 61: | ||
*The reported adverse events may not represent the highly concerning ones. | *The reported adverse events may not represent the highly concerning ones. | ||
==Funding== | ==Funding== | ||
*Merit Review Award from the US Department of Veterans Affairs Health Services Research and Development Service | |||
==See Also== | ==See Also== | ||
*[[Opioid Deprescribing]] | *[[Opioid Deprescribing]] | ||
[[Category:EBQ]] | [[Category:EBQ]] | ||
Revision as of 19:07, 26 April 2021
Krebs et al.. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA 2018. 319:872-882. PMID: 29509867. DOI. Full Text.
Clinical Question
To compare opioid vs nonopioid medications over 12 months on pain-related function, pain intensity, and adverse effects in patients with moderate to severe chronic back or osteoarthritic hip/knee pain.
Bottom Line
In patients with severe back or hip/knee pain that weren't currently receiving opioid treatment, there was no difference between opioid vs. non-opioid treatment over 12 months with an escalating treatment-to-target approach. There may be more adverse events with opioid therapy.
Study Design
- Pragmatic, single-centre, open label, randomized trial
- N=240
- Opioid (n=120)
- Non-opioid (n=120)
- Setting: 62 American primary care clinicians affiliated with the Veterans Affairs
- Enrolment: June 2013 to December 2015
- Mean follow-up: 12 months
- Analysis: Intention-to-treat, masked outcome assessment.
- Primary Outcome: Improvement in pain-related function assessed with the Brief Pain Inventory (BPI)
Population Studied
Inclusion Criteria
- Back pain or knee or hip pain associated with osteoarthritis
- Pain occurring almost every day for ≥6 months despite non-opioid analgesic use
- Brief Pain Inventory (BPI) scale average pain ≥5 and BPI interference score ≥5
Exclusion Criteria
- Severe mental illnesses
- Moderate severe cognitive function
- Planned surgery to address pain in the next year
- Long term opioid treatment
- Contraindications to study medications
- Life expectancy <12 months
Baseline Characteristics
- Demographics: Mean age 57 years, 13% female, 88% white, 6% black, 6% other ethnicity, higher education 24%
- Employment: Employed 42%, self-employed 6%, retired 36%
- Pain eligibility: Back pain 65%, Hip/knee osteoarthritis pain 35%
- Smoker 21%, hazardous alcohol use (AUDIT Score ≥8) 3%, illicit drug use in prior year 7%
- Mental Health: Moderate depression 23%, Moderate anxiety 9%, PTSD 21%
Interventions
Participants were randomized to a group in and received stepwise approach to management of pain:
- Opioid Group - Titrated to maximum of 100 morphine-equivalent mg:
- Morphine IR, hydrocodone/acetaminophen, oxycodone IR
- Morphine sustained release, oxycodone sustained release
- Transdermal fentanyl
- Non-opioid
- Acetaminophen and NSAIDs
- Adjuvant oral medications (nortriptyline, amitriptyline, gabapentin), topical analgesics (capsaicin, lidocaine)
- Drugs requiring pre-authorization in VA system (pregabalin, duloxetine, tramadol)
Outcomes/Results
Primary Outcomes
Secondary Outcomes
Adverse events
Discussion
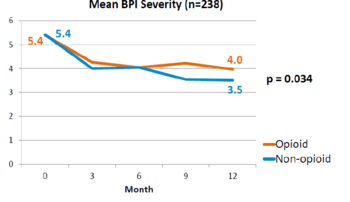
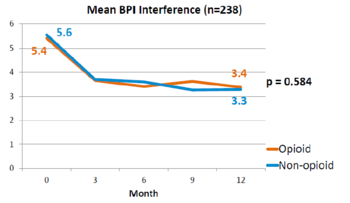
This is a landmark study published in 2018 by Krebs et al - the SPACE study. It was a pragmatic randomised controlled trial comparing opioid versus non opioid analgesics for 12 months in primary care. Participants were 240 VA patients with moderate to severe chronic back pain or knee/hip OA, and not on opioids. The mean pain intensity initially was 5.4 in both arms. Pain scores at 1 year was worse in the opioid arm (4.0) than non opioid (3.5) (P=0.034). There was no difference in pain interference, and adverse effects were worse in opioid group (P=0.03).
Criticism
- Unblinded
- Patient self-reporting is an area of bias
- The patients were veterans, and so this might limit the external validity
- Patients using opioids already were excluded
- The reported adverse events may not represent the highly concerning ones.
Funding
- Merit Review Award from the US Department of Veterans Affairs Health Services Research and Development Service