Gabapentinoids: Difference between revisions
From WikiMSK
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
==Gabapentin== | |||
* First discovered in 1970s in an attempt to create a GABA analogue | * First discovered in 1970s in an attempt to create a GABA analogue | ||
* Whilst it resembles GABA, it does not act on the GABA receptor. | * Whilst it resembles GABA, it does not act on the GABA receptor. | ||
| Line 14: | Line 13: | ||
** Elimination: Renal excretion, half life 5-7 hours. Dose adjustment in renal impairment | ** Elimination: Renal excretion, half life 5-7 hours. Dose adjustment in renal impairment | ||
==Pregabalin== | |||
* Similar to gabapentin. Binds to α2δ subunits of voltage-dependent calcium channels to reduce calcium influx | * Similar to gabapentin. Binds to α2δ subunits of voltage-dependent calcium channels to reduce calcium influx | ||
| Line 27: | Line 25: | ||
==Recommended prescribing: NZF== | |||
Gabapentin | '''Gabapentin''' | ||
* Day 1 300mg nocte | * Day 1 300mg nocte | ||
| Line 36: | Line 34: | ||
* Then increase by 300mg every 2-3 days to max dose 3600mg daily | * Then increase by 300mg every 2-3 days to max dose 3600mg daily | ||
Pregabalin | '''Pregabalin''' | ||
* Initially 75mg bd | * Initially 75mg bd | ||
* 150mg bd after 3-7 days | * 150mg bd after 3-7 days | ||
| Line 47: | Line 46: | ||
Caution in pregnancy (category B1); no clear data available, use if benefits outweigh risks | Caution in pregnancy (category B1); no clear data available, use if benefits outweigh risks | ||
==Evidence== | |||
''' | '''Post-herpetic neuralgia, diabetic peripheral neuropathy and fibromyalgia | ||
Post-herpetic neuralgia, diabetic peripheral neuropathy and fibromyalgia | ''' | ||
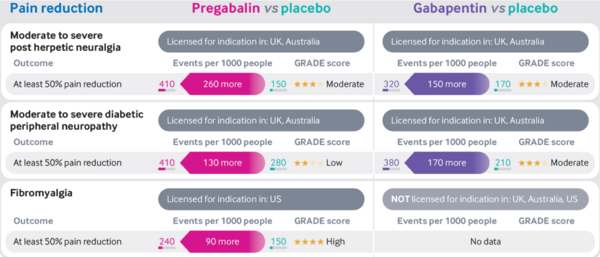
* Moderate quality evidence supports the use of gabapentinoids to improve pain in those with post-herpetic neuralgia or diabetic peripheral neuropathy compared with placebo <ref>{{#pmid:30673120}}</ref> <ref>{{#pmid:28597471}}</ref> | * Moderate quality evidence supports the use of gabapentinoids to improve pain in those with post-herpetic neuralgia or diabetic peripheral neuropathy compared with placebo <ref>{{#pmid:30673120}}</ref> <ref>{{#pmid:28597471}}</ref> | ||
* High quality evidence supports the use of pregabalin to improve pain in those with fibromyalgia compared to placebo <ref>{{#pmid:27684492}}</ref> | * High quality evidence supports the use of pregabalin to improve pain in those with fibromyalgia compared to placebo <ref>{{#pmid:27684492}}</ref> | ||
* The evidence for gabapentin in fibromyalgia is unclear because of the small number of trials and very low quality of evidence available <ref>{{#pmid:28045473}}</ref> | * The evidence for gabapentin in fibromyalgia is unclear because of the small number of trials and very low quality of evidence available <ref>{{#pmid:28045473}}</ref> | ||
* NNTs | |||
** Moderate to severe post-herpetic neuralgia: Pregabalin 4, Gabapentin 7 | |||
** Moderate to severe diabetic peripheral neuropathy: Pregabalin 8, Gabapentin 6 | |||
** Fibromyalgia: Gabapentin 10 | |||
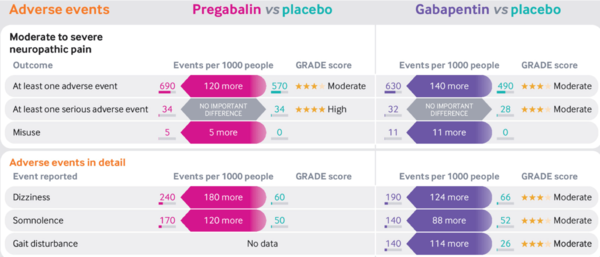
* NNHs | |||
** Moderate to severe neuropathic pain: Pregabalin 8, Gabapentin 7 | |||
Low back and radicular pain | '''Low back and radicular pain''' | ||
* Systematic review and meta-analysis of 7 RCTs compared gabapentin and pregabalin to placebo. Judged moderate-high quality data <ref>{{#pmid:29970367}}</ref> | * Systematic review and meta-analysis of 7 RCTs compared gabapentin and pregabalin to placebo. Judged moderate-high quality data <ref>{{#pmid:29970367}}</ref> | ||
* Low back pain with or without lumbar radicular pain | * Low back pain with or without lumbar radicular pain | ||
| Line 60: | Line 65: | ||
* Lumbar radicular pain only | * Lumbar radicular pain only | ||
** No difference in pain or disability at short, intermediate or long term follow up | ** No difference in pain or disability at short, intermediate or long term follow up | ||
[[File:Gabapentinoids vs placebo pain infographic Mathieson.png|600px]] | |||
[[File:Gabapentinoids vs placebo adverse effects infographic Mathieson.png|600px]] | |||
==References== | |||
Revision as of 08:45, 25 June 2021
Gabapentin
- First discovered in 1970s in an attempt to create a GABA analogue
- Whilst it resembles GABA, it does not act on the GABA receptor.
- Later discovered to act on α2δ subunits of voltage-dependent calcium channels to reduce calcium influx
- Precise mechanism of analgesia unclear
- Inhibits release of excitatory neurotransmitters: glutamate, NA, substance P
- Medsafe licenced for: neuropathic pain, adjunct anti-epileptic
- Pharmacokinetics
- Absorption: Saturable transporter so delayed peak levels at higher doses. Drugs that reduce motility (e.g opiates) increase bioavailability. Peak serum conc 3 hours
- Distribution: Less lipophilic so requires active transport across the BBB
- Metabolism: minimal
- Elimination: Renal excretion, half life 5-7 hours. Dose adjustment in renal impairment
Pregabalin
- Similar to gabapentin. Binds to α2δ subunits of voltage-dependent calcium channels to reduce calcium influx
- Inhibits release of excitatory neurotransmitters: glutamate, NA, substance P
- Medsafe licenced for: neuropathic pain, adjunct anti-epileptic
- Pharmacokinetics
- Absorption: Rapid absorption after oral administration. Peak serum conc 1h
- Distribution: Less lipophilic so requires active transport across the BBB
- Metabolism: minimal, no active metabolites
- Elimination: Renal excretion, half life 6.3 hours. Dose adjustment in renal impairment
Recommended prescribing: NZF
Gabapentin
- Day 1 300mg nocte
- Day 2 300mg bd
- Day 3 300mg tds
- Then increase by 300mg every 2-3 days to max dose 3600mg daily
Pregabalin
- Initially 75mg bd
- 150mg bd after 3-7 days
- Max dose 300mg bd after further 7 days
Titrate upwards until pain relief, side effects, or max dose reached
Remember to dose adjust for renal impairment: gabapentin if <80mL/min, pregabalin if <60mL/min
Caution in pregnancy (category B1); no clear data available, use if benefits outweigh risks
Evidence
Post-herpetic neuralgia, diabetic peripheral neuropathy and fibromyalgia
- Moderate quality evidence supports the use of gabapentinoids to improve pain in those with post-herpetic neuralgia or diabetic peripheral neuropathy compared with placebo [1] [2]
- High quality evidence supports the use of pregabalin to improve pain in those with fibromyalgia compared to placebo [3]
- The evidence for gabapentin in fibromyalgia is unclear because of the small number of trials and very low quality of evidence available [4]
- NNTs
- Moderate to severe post-herpetic neuralgia: Pregabalin 4, Gabapentin 7
- Moderate to severe diabetic peripheral neuropathy: Pregabalin 8, Gabapentin 6
- Fibromyalgia: Gabapentin 10
- NNHs
- Moderate to severe neuropathic pain: Pregabalin 8, Gabapentin 7
Low back and radicular pain
- Systematic review and meta-analysis of 7 RCTs compared gabapentin and pregabalin to placebo. Judged moderate-high quality data [5]
- Low back pain with or without lumbar radicular pain
- No difference in pain or disability at short, intermediate or long term follow up
- Lumbar radicular pain only
- No difference in pain or disability at short, intermediate or long term follow up
References
- ↑ Derry et al.. Pregabalin for neuropathic pain in adults. The Cochrane database of systematic reviews 2019. 1:CD007076. PMID: 30673120. DOI. Full Text.
- ↑ Wiffen et al.. Gabapentin for chronic neuropathic pain in adults. The Cochrane database of systematic reviews 2017. 6:CD007938. PMID: 28597471. DOI. Full Text.
- ↑ Derry et al.. Pregabalin for pain in fibromyalgia in adults. The Cochrane database of systematic reviews 2016. 9:CD011790. PMID: 27684492. DOI. Full Text.
- ↑ Cooper et al.. Gabapentin for fibromyalgia pain in adults. The Cochrane database of systematic reviews 2017. 1:CD012188. PMID: 28045473. DOI. Full Text.
- ↑ Enke et al.. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2018. 190:E786-E793. PMID: 29970367. DOI. Full Text.