Paroxysmal Extreme Pain Disorder: Difference between revisions
No edit summary |
mNo edit summary |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Authors}} | {{Authors | ||
|Authors=Jeremy | |||
}} | |||
{{Condition | {{Condition | ||
|quality=Partial | |||
|image=PEPD flushing.jpg | |||
|inheritance=Autosomal dominant | |inheritance=Autosomal dominant | ||
|genetics=SCN9A gain of function mutation | |genetics=SCN9A gain of function mutation | ||
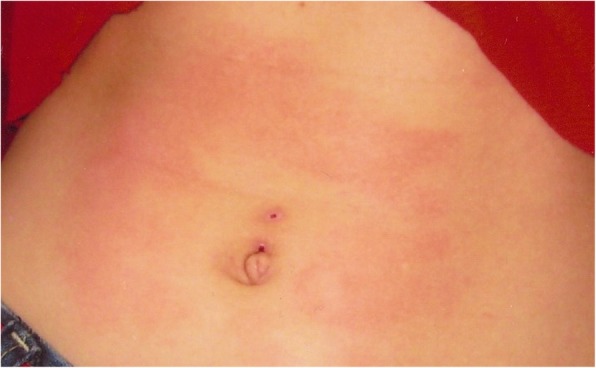
|clinicalfeatures=Attacks of rectal, ocular, and mandibular pain associated with skin flushing. | |||
}} | }} | ||
Paroxysmal Extreme Pain Disorder is a | Paroxysmal Extreme Pain Disorder (PEPD) is a rare [[Sodium Channelopathies|sodium channelopathy]] resulting from a gain-of-function mutation in the SCN9A gene. This condition is characterized by episodes of severe pain in the rectal, ocular, or submandibular regions, accompanied by skin erythema. Typically, the onset occurs during infancy or the neonatal period. It was previously referred to as familial rectal pain. | ||
== | == Epidemiology == | ||
Paroxysmal extreme pain disorder is caused by | PEPD is a rare disorder, with only 500 cases reported in the literature. It is likely frequently misdiagnosed and underdiagnosed.<ref>{{Cite journal|last=Stępień|first=Adam|last2=Sałacińska|first2=Daria|last3=Staszewski|first3=Jacek|last4=Durka-Kęsy|first4=Marta|last5=Dobrogowski|first5=Jan|date=2020-05-13|title=Paroxysmal extreme pain disorder in family with c.3892G > T (p.Val1298Phe) in the SCN9A gene mutation - case report|url=https://pubmed.ncbi.nlm.nih.gov/32404070|journal=BMC neurology|volume=20|issue=1|pages=182|doi=10.1186/s12883-020-01770-9|issn=1471-2377|pmc=7218613|pmid=32404070}}</ref> | ||
== Genetics and Pathophysiology == | |||
PEPD is caused by a gain-of-function mutation in the SCN9A gene, which encodes the alpha subunit of a voltage-gated sodium channel called Nav1.7. These sodium channels are found in peripheral somatic and visceral sensory nerves, nociceptors, dorsal root ganglia, sympathetic ganglia, trigeminal ganglia, and olfactory cells. | |||
The penetrance in families with SCN9A paroxysmal extreme pain disorder reported to date is 100%. | The penetrance in families with SCN9A paroxysmal extreme pain disorder reported to date is 100%. | ||
== Pathophysiology == | |||
The Nav1.7 channel plays a crucial role in generating and conducting nerve impulses (action potentials), particularly in pain-sensing neurons (nociceptors). Mutations in the SCN9A gene lead to a gain of function, causing the Nav1.7 channel to open more easily and amplify pain signals. This results in the heightened pain sensitivity observed in individuals with PEPD. | |||
== Clinical Features == | == Clinical Features == | ||
The disorder is often characterized by: | The disorder is often characterized by: | ||
* Autonomic manifestations during infancy, including skin flushing, harlequin (patchy or asymmetric) | * Autonomic manifestations during infancy, including skin flushing, harlequin (patchy or asymmetric) colour change, tonic non-epileptic attacks (stiffening), and syncope with bradycardia. | ||
* Older children and adults describe the pain as excruciating, burning, or sharp. | * Older children and adults describe the pain as excruciating, burning, or sharp. | ||
* Pain that is initially localized but spreads during severe attacks (e.g., from the rectum to the abdomen). | * Pain that is initially localized but spreads during severe attacks (e.g., from the rectum to the abdomen). | ||
* Attacks lasting from seconds to | * Attacks lasting from seconds to several hours. | ||
* Constipation between episodes due to reluctance to pass stool and precipitate a painful attack. | * Constipation between episodes due to reluctance to pass stool and precipitate a painful attack. | ||
* The number of rectal attacks can decrease with age, whereas ocular and jaw attacks may increase with age. | * The number of rectal attacks can decrease with age, whereas ocular and jaw attacks may increase with age. | ||
| Line 32: | Line 43: | ||
* Cardiac arrhythmia: Bradycardia and even asystole during atonic attacks may prompt evaluation for a primary cardiac cause. | * Cardiac arrhythmia: Bradycardia and even asystole during atonic attacks may prompt evaluation for a primary cardiac cause. | ||
*Gastrointestinal reflux: Pain, facial erythema, and crying after eating may resemble gastroesophageal reflux.|ddx-title=Differential diagnosis of SCN9A paroxysmal extreme pain disorder includes:}} | *Gastrointestinal reflux: Pain, facial erythema, and crying after eating may resemble gastroesophageal reflux.|ddx-title=Differential diagnosis of SCN9A paroxysmal extreme pain disorder includes:}} | ||
== Diagnosis == | |||
Genetic testing can confirm a diagnosis of PEPD in individuals who have clinical signs and symptoms of the disorder. Testing involves sequencing the SCN9A gene to identify any pathogenic variants. As the disorder follows an autosomal dominant inheritance pattern, a family history of PEPD may be present. | |||
== Management == | == Management == | ||
There is currently no cure for the disorder, and management is focused on alleviating symptoms and preventing triggers. | |||
=== | === General Treatment === | ||
* Use stool softeners | * To reduce the likelihood of triggering an attack: Use stool softeners and pass stool slowly | ||
* | * Pelvic floor muscle exercises and prevention of constipation have been described as beneficial in the literature. | ||
=== Medication === | === Medication === | ||
* Carbamazepine is the most effective (though not completely effective) treatment for reducing the number and severity of PEPD attacks.<ref>{{Cite journal|last=Fertleman|first=C. R.|last2=Ferrie|first2=C. D.|last3=Aicardi|first3=J.|last4=Bednarek|first4=N. a. F.|last5=Eeg-Olofsson|first5=O.|last6=Elmslie|first6=F. V.|last7=Griesemer|first7=D. A.|last8=Goutières|first8=F.|last9=Kirkpatrick|first9=M.|last10=Malmros|first10=I. N. O.|last11=Pollitzer|first11=M.|date=2007-08-07|title=Paroxysmal extreme pain disorder (previously familial rectal pain syndrome)|url=https://pubmed.ncbi.nlm.nih.gov/17679678|journal=Neurology|volume=69|issue=6|pages=586–595|doi=10.1212/01.wnl.0000268065.16865.5f|issn=1526-632X|pmid=17679678}}</ref> | * [[Carbamazepine]] is the most effective (though not completely effective) treatment for reducing the number and severity of PEPD attacks.<ref>{{Cite journal|last=Fertleman|first=C. R.|last2=Ferrie|first2=C. D.|last3=Aicardi|first3=J.|last4=Bednarek|first4=N. a. F.|last5=Eeg-Olofsson|first5=O.|last6=Elmslie|first6=F. V.|last7=Griesemer|first7=D. A.|last8=Goutières|first8=F.|last9=Kirkpatrick|first9=M.|last10=Malmros|first10=I. N. O.|last11=Pollitzer|first11=M.|date=2007-08-07|title=Paroxysmal extreme pain disorder (previously familial rectal pain syndrome)|url=https://pubmed.ncbi.nlm.nih.gov/17679678|journal=Neurology|volume=69|issue=6|pages=586–595|doi=10.1212/01.wnl.0000268065.16865.5f|issn=1526-632X|pmid=17679678}}</ref> | ||
* Additional anti-seizure medications, such as lamotrigine, topiramate, tiagabine, and sodium valproate, have been reported to have varying effectiveness. | * Additional anti-seizure medications, such as lamotrigine, topiramate, tiagabine, and sodium valproate, have been reported to have varying effectiveness. | ||
* Analgesics and opiates are ineffective in the treatment of PEPD. | * Analgesics and opiates are ineffective in the treatment of PEPD. | ||
* In children experiencing severe attacks, a 1:1 mixture of nitrous oxide and oxygen may provide relief. | |||
== Resources == | == Resources == | ||
| Line 51: | Line 66: | ||
== References == | == References == | ||
[[Category:Genetic Disorders]] | [[Category:Genetic Disorders]] | ||
{{References}} | {{References}} | ||
{{Reliable sources}} | {{Reliable sources}} | ||
Latest revision as of 16:09, 23 March 2023
Paroxysmal Extreme Pain Disorder (PEPD) is a rare sodium channelopathy resulting from a gain-of-function mutation in the SCN9A gene. This condition is characterized by episodes of severe pain in the rectal, ocular, or submandibular regions, accompanied by skin erythema. Typically, the onset occurs during infancy or the neonatal period. It was previously referred to as familial rectal pain.
Epidemiology
PEPD is a rare disorder, with only 500 cases reported in the literature. It is likely frequently misdiagnosed and underdiagnosed.[1]
Genetics and Pathophysiology
PEPD is caused by a gain-of-function mutation in the SCN9A gene, which encodes the alpha subunit of a voltage-gated sodium channel called Nav1.7. These sodium channels are found in peripheral somatic and visceral sensory nerves, nociceptors, dorsal root ganglia, sympathetic ganglia, trigeminal ganglia, and olfactory cells.
The penetrance in families with SCN9A paroxysmal extreme pain disorder reported to date is 100%.
Pathophysiology
The Nav1.7 channel plays a crucial role in generating and conducting nerve impulses (action potentials), particularly in pain-sensing neurons (nociceptors). Mutations in the SCN9A gene lead to a gain of function, causing the Nav1.7 channel to open more easily and amplify pain signals. This results in the heightened pain sensitivity observed in individuals with PEPD.
Clinical Features
The disorder is often characterized by:
- Autonomic manifestations during infancy, including skin flushing, harlequin (patchy or asymmetric) colour change, tonic non-epileptic attacks (stiffening), and syncope with bradycardia.
- Older children and adults describe the pain as excruciating, burning, or sharp.
- Pain that is initially localized but spreads during severe attacks (e.g., from the rectum to the abdomen).
- Attacks lasting from seconds to several hours.
- Constipation between episodes due to reluctance to pass stool and precipitate a painful attack.
- The number of rectal attacks can decrease with age, whereas ocular and jaw attacks may increase with age.
PEPD episodes can be triggered by:
- Defecation or perineal wiping (rectal attacks)
- Eating (jaw attacks)
- Cold wind, temperature change, or crying (ocular attacks)
Differential Diagnosis
- Epilepsy: Atonic attacks in infants can resemble epileptic seizures, but EEG during attacks shows slowing without epileptiform activity.
- Hereditary Hyperekplexia
- Cardiac arrhythmia: Bradycardia and even asystole during atonic attacks may prompt evaluation for a primary cardiac cause.
- Gastrointestinal reflux: Pain, facial erythema, and crying after eating may resemble gastroesophageal reflux.
Diagnosis
Genetic testing can confirm a diagnosis of PEPD in individuals who have clinical signs and symptoms of the disorder. Testing involves sequencing the SCN9A gene to identify any pathogenic variants. As the disorder follows an autosomal dominant inheritance pattern, a family history of PEPD may be present.
Management
There is currently no cure for the disorder, and management is focused on alleviating symptoms and preventing triggers.
General Treatment
- To reduce the likelihood of triggering an attack: Use stool softeners and pass stool slowly
- Pelvic floor muscle exercises and prevention of constipation have been described as beneficial in the literature.
Medication
- Carbamazepine is the most effective (though not completely effective) treatment for reducing the number and severity of PEPD attacks.[2]
- Additional anti-seizure medications, such as lamotrigine, topiramate, tiagabine, and sodium valproate, have been reported to have varying effectiveness.
- Analgesics and opiates are ineffective in the treatment of PEPD.
- In children experiencing severe attacks, a 1:1 mixture of nitrous oxide and oxygen may provide relief.
Resources
GeneReviews - SCN9A Neuropathic Pain Syndromes
References
- ↑ Stępień, Adam; Sałacińska, Daria; Staszewski, Jacek; Durka-Kęsy, Marta; Dobrogowski, Jan (2020-05-13). "Paroxysmal extreme pain disorder in family with c.3892G > T (p.Val1298Phe) in the SCN9A gene mutation - case report". BMC neurology. 20 (1): 182. doi:10.1186/s12883-020-01770-9. ISSN 1471-2377. PMC 7218613. PMID 32404070.
- ↑ Fertleman, C. R.; Ferrie, C. D.; Aicardi, J.; Bednarek, N. a. F.; Eeg-Olofsson, O.; Elmslie, F. V.; Griesemer, D. A.; Goutières, F.; Kirkpatrick, M.; Malmros, I. N. O.; Pollitzer, M. (2007-08-07). "Paroxysmal extreme pain disorder (previously familial rectal pain syndrome)". Neurology. 69 (6): 586–595. doi:10.1212/01.wnl.0000268065.16865.5f. ISSN 1526-632X. PMID 17679678.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,