Radiation Safety: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
Early transient erythema (dose >2000 mGy) appears a few hours after irradiation. It is an inflammatory response resulting in increased permeability of capillaries. This resolves over a few days even after high doses. It will always occur for doses much greater than 2000 mGy so it is an early indication that more significant skin effects are possible. Main Erythema (dose > 6000 mGy) appears around 10 days after irradiation. The rate of cell loss is independent of dose. Dermal necrosis (>18000 mGy) takes around 10 weeks to occur. Sicker patients will tend to receive higher doses with procedures. We work on the basis that effects are possible at low doses. | Early transient erythema (dose >2000 mGy) appears a few hours after irradiation. It is an inflammatory response resulting in increased permeability of capillaries. This resolves over a few days even after high doses. It will always occur for doses much greater than 2000 mGy so it is an early indication that more significant skin effects are possible. Main Erythema (dose > 6000 mGy) appears around 10 days after irradiation. The rate of cell loss is independent of dose. Dermal necrosis (>18000 mGy) takes around 10 weeks to occur. Sicker patients will tend to receive higher doses with procedures. We work on the basis that effects are possible at low doses. | ||
Populations exposed to radiation show an increase in cancer incidence. There is no unique radiation cancers. The risk of induction varies with organ. There is a delay before the increase is seen (a few years for leukaemia and bone tumours, and 20+ years for most solid cancers). There have been many epidemiology studies trying to evaluate the dose threshold. For example the Life Span Study (A-bomb survivers), was last reviewed in 1996. There were an estimated 420 excess cancer deaths among the 86,572 survivors within 2.5km of the epicentre. | ==Cancer Risk Estimates== | ||
Populations exposed to radiation show an increase in cancer incidence. There is no unique radiation cancers. The risk of induction varies with organ. There is a delay before the increase is seen (a few years for leukaemia and bone tumours, and 20+ years for most solid cancers). There have been many epidemiology studies trying to evaluate the dose threshold. For example the Life Span Study (A-bomb survivers), was last reviewed in 1996. There were an estimated 420 excess cancer deaths among the 86,572 survivors within 2.5km of the epicentre. There are complications with estimating risks of radiation induced cancer. The four complications are going from high to low doses, going from high to low dose rates, projecting hte risk to full lifetime, and transferring the risk estimates across populations. Other questions include whether there is a threshold, and what is the shape of the relationship between cancer risk and dose - is it linear, linear-quadratic. | |||
The ICRP 103 published in 2007 estimated risk at 11% fatal risk per Sv with exposure to x-rays at high doses and dose rates. It is assumed that there is no threshold. Allowance is made for low doses-dose rates - 5% fatal risk per Sv at low doses/dose rates. | |||
==Radiation Protection== | |||
There is the Radiation Safety Act 2016. Under the act there are regulations outlining who can use radiating materials without a license (e.g. radiologists), and who needs a license. Whoever owns the x-ray system has legal responsibilities that it is used competently. The Codes of practice (C1 2018) are the code of practice for diagnostic and interventional radiology. The key concepts are Justication, Optimisation, and Limitation. For limiting cancers and hereditary effects the limits for occupational exposure are 20mSv per year over any 5 year period, and not more than 50mSv in any one year. While for the public is 1mSv per year. For preventing deterministic effects - 20mSv for eye, 500mSv for skin, and 500mSv for hands and feet for occupational exposure. | |||
X-ray scatter comes off the patient, which is the source of occupational exposure. We are working with the primary beam and to reduce scatter. For a given source of scatter consider distance, time, and shielding. These factors are used to minimise occupational exposure. The patient is the source of scatter, and the intensity of scatter depends on the intensity of the primary beam. Rule of thumb is that the intensity of scatter at 1 metre from the patient is about 1/1000 of that in the incident primary beam. The first few centimetres is where the dose is most intense. If you are between the x-ray beam and patient then the dose is reduced, so stay away from the zone between the x-ray tube and patient. i.e. have the tube below the patient, and image detector above the patient. | |||
Be aware of where people are standing, and use a little bit of distance as much as possible. Minimise the time spent in areas where scatter is present. Only be in the x-ray room or theatre if required. Minimise the "beam on" time in fluoroscopy, use the last image hold. | |||
Shielding is in walls, doors, windows, ceilings, floors. There are permanent operator barriers. Use protective clothing - lead aprons/coats (there are differences in thickness, be aware that the stated lead rating may only apply to the overlap region). The size of the aprons is very important, if it is too small that will reduce the protection due to reduced overlap. Consider lead glasses in certain cases. Lead gloves are not often used. Thyroid shields are normally a standard part of protective equipment. Movable barriers can be useful. 2mm of lead can stop all beams which is too thick to be worn but not too thick for a movable barrier and so has the potential to give further protection. Lead aprons are typically 90-97% effective depending on thickness (0.3-0.5mm thick). Lead glasses reduce eye dose around 50%. | |||
There is an effect of scatter based on patient size. X-ray beam area - scatter is proportional to beam area. Collimation leads to lower patient dose, lower occupational dose, and better image quality. Low patient dose means low occupational dose. Also be aware how the equipment is set up by the manufacturer. All equipment needs to be checked yearly. The software has a large bearing on the dose of the system. | |||
You are required to be monitored with occupational doses. Personal monitoring with retrospective dosimeter/live monitoring. There should be a personal dosimeter attached to the C-arm. There are many challenges with staff monitoring. Monitoring confirms good practice. | |||
[[Category:Radiology]] | [[Category:Radiology]] | ||
Revision as of 07:58, 4 March 2021
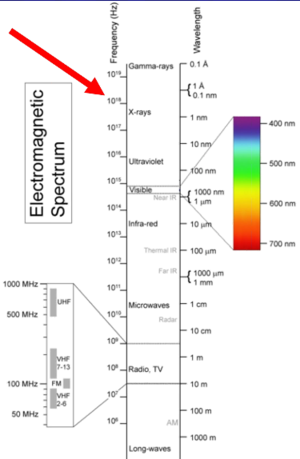
X-rays are on the electromagnetic spectrum, they are massless particles that travel at the speed of light. They have a very short wavelength which gives it certain properties. You can't see it, it passes through things, and it ionizes all matter. In a biological system it can give rise to unwanted effects. The x-ray scatter is very similar to visible light scatter. Think of x-rays of short wavelength light that is ionizing.
Every x-ray machine uses the same fundamental method of producing x-rays. It is measured in volts (kVs), and milliamps. The focal spot is very small - generated from a point. The intensity drops off over the square of the distance (1 / r^2). This is called the inverse square law related to divergence. Getting closer to the source causing a rapid rise in intensity. X-rays interact with matter via ionisation, and the intensity reduces exponentially reduces due to attenuation. These two phenomenon - divergence and attenuation - can be used to improve safety.
X-ray beams can be measured. Exposure is the easiest thing to measure and is the charge per mass in air. Fluoroscopy systems have an air chamber which collects the amount of charge in that small volume of air, and the ionization of air is measured. The absorbed dose related to the energy per mass. It is measured in Joules per kilogram in Grays (Gy). Systems will measure this in milli-grays. An orthopaedic type procedure may be around 100mGy, while a radiotherapy dose may be around 50Gy. The equivalent dose is the biologically weighted absorbed dose and is measured in Sievert (Sv). This relates to the overall risk to the patient. For x-rays 1 Gy = 1 Sv (whole body exposure). Ethics risk calculations and occupational exposure calculations use Sieverts.
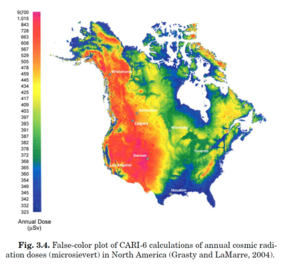
Natural background sources give around 2mSv. There is cosmic radiation, internal radiation, radon, mildly radioactive food. There is a slight variation in altitude with higher doses at higher altitudes. A patient dose is typically in 0.05-20mSv. Long cases may get up to 20mSv. Multi-phase CT scans can also get up to 20mSv. One of the challenges is that in over 100mSv as a single dose there is an increased risk of cancers. We live in this uncertain zone where we are working in a low level where we are not completely sure what the risk is.
Occupational exposure is similar to moving from a low altitude to a higher altitude area. Reducing population dose is focused on diagnostic procedures.
The total energy absorbed from x-rays) even at LD50 is very small. It is the microscopic energy deposition that is important (DNA the principal target). The repair of damage is very sophisticated (background radiation). Total repair leads to no effects. While mis-repaired or unrepaired damage leads to cell death (deterministic effects), or the cell can remain viable but have stochastic effects (cancer for somatic cells, and hereditary effects on germ cells). With the stochastic effects you can't always predict the effect. These effects have a threshold with effects not occurring below the threshold.
The organs and tissues of concerns are skin damage, cataracts, reduced IQ to the embryo, and impaired fertility with damage to gonads. We are starting to see these effects in dedicated rooms. There has been an increasing number of long complex x-rays, and increased capacity of modern x-ray tubes to produce x-rays. A couple of decades ago the tubes would overheat before excessive radiation production, but they are now oil cooled which has stopped this thermal limit. Some interventional fellows are reaching levels of over 0.15Gy per years which can cause cataracts. >0.15Gy can cause temporary sterility in testes. 4-6Gy can cause permanent sterility in testes, and 3-6Gy in ovaries. Transient erythema is the first effect, and the dose needs to get to 2Gy. Normal fluoroscopy is 50mGy per minute, so 40 minutes is needed to get transient erythema. Main erythema occurs at 6Gy, dermal atrophy at 11Gy, moist desquamation at 15Gy, and dermal necrosis at 18Gy. The repair process is quite quick, i.e. the effect is not accumulative over days. Fluoroscopy systems also will tell you the dose per area but this can lead to confusion.
Early transient erythema (dose >2000 mGy) appears a few hours after irradiation. It is an inflammatory response resulting in increased permeability of capillaries. This resolves over a few days even after high doses. It will always occur for doses much greater than 2000 mGy so it is an early indication that more significant skin effects are possible. Main Erythema (dose > 6000 mGy) appears around 10 days after irradiation. The rate of cell loss is independent of dose. Dermal necrosis (>18000 mGy) takes around 10 weeks to occur. Sicker patients will tend to receive higher doses with procedures. We work on the basis that effects are possible at low doses.
Cancer Risk Estimates
Populations exposed to radiation show an increase in cancer incidence. There is no unique radiation cancers. The risk of induction varies with organ. There is a delay before the increase is seen (a few years for leukaemia and bone tumours, and 20+ years for most solid cancers). There have been many epidemiology studies trying to evaluate the dose threshold. For example the Life Span Study (A-bomb survivers), was last reviewed in 1996. There were an estimated 420 excess cancer deaths among the 86,572 survivors within 2.5km of the epicentre. There are complications with estimating risks of radiation induced cancer. The four complications are going from high to low doses, going from high to low dose rates, projecting hte risk to full lifetime, and transferring the risk estimates across populations. Other questions include whether there is a threshold, and what is the shape of the relationship between cancer risk and dose - is it linear, linear-quadratic.
The ICRP 103 published in 2007 estimated risk at 11% fatal risk per Sv with exposure to x-rays at high doses and dose rates. It is assumed that there is no threshold. Allowance is made for low doses-dose rates - 5% fatal risk per Sv at low doses/dose rates.
Radiation Protection
There is the Radiation Safety Act 2016. Under the act there are regulations outlining who can use radiating materials without a license (e.g. radiologists), and who needs a license. Whoever owns the x-ray system has legal responsibilities that it is used competently. The Codes of practice (C1 2018) are the code of practice for diagnostic and interventional radiology. The key concepts are Justication, Optimisation, and Limitation. For limiting cancers and hereditary effects the limits for occupational exposure are 20mSv per year over any 5 year period, and not more than 50mSv in any one year. While for the public is 1mSv per year. For preventing deterministic effects - 20mSv for eye, 500mSv for skin, and 500mSv for hands and feet for occupational exposure.
X-ray scatter comes off the patient, which is the source of occupational exposure. We are working with the primary beam and to reduce scatter. For a given source of scatter consider distance, time, and shielding. These factors are used to minimise occupational exposure. The patient is the source of scatter, and the intensity of scatter depends on the intensity of the primary beam. Rule of thumb is that the intensity of scatter at 1 metre from the patient is about 1/1000 of that in the incident primary beam. The first few centimetres is where the dose is most intense. If you are between the x-ray beam and patient then the dose is reduced, so stay away from the zone between the x-ray tube and patient. i.e. have the tube below the patient, and image detector above the patient.
Be aware of where people are standing, and use a little bit of distance as much as possible. Minimise the time spent in areas where scatter is present. Only be in the x-ray room or theatre if required. Minimise the "beam on" time in fluoroscopy, use the last image hold.
Shielding is in walls, doors, windows, ceilings, floors. There are permanent operator barriers. Use protective clothing - lead aprons/coats (there are differences in thickness, be aware that the stated lead rating may only apply to the overlap region). The size of the aprons is very important, if it is too small that will reduce the protection due to reduced overlap. Consider lead glasses in certain cases. Lead gloves are not often used. Thyroid shields are normally a standard part of protective equipment. Movable barriers can be useful. 2mm of lead can stop all beams which is too thick to be worn but not too thick for a movable barrier and so has the potential to give further protection. Lead aprons are typically 90-97% effective depending on thickness (0.3-0.5mm thick). Lead glasses reduce eye dose around 50%.
There is an effect of scatter based on patient size. X-ray beam area - scatter is proportional to beam area. Collimation leads to lower patient dose, lower occupational dose, and better image quality. Low patient dose means low occupational dose. Also be aware how the equipment is set up by the manufacturer. All equipment needs to be checked yearly. The software has a large bearing on the dose of the system.
You are required to be monitored with occupational doses. Personal monitoring with retrospective dosimeter/live monitoring. There should be a personal dosimeter attached to the C-arm. There are many challenges with staff monitoring. Monitoring confirms good practice.