Scheuermann's Disease
Scheuermann's disease (SD) is a developmental disorder in adolescence that causes a rigid or relatively rigid hyperkyphosis of the thoracic or thoracolumbar spine and has specific radiographic findings. It most commonly occurs in the adolescent growth spurt between the ages of 12 and 15, but can occur as early as late preschool.[1]
Classification
There are two curve patterns. [1][2]
- Type I: Also known as Classic Scheuermann's Disease. There is a hyperkyphosis due to wedge vertebrae with the apex of the curve found at the mid thoracic spine, lower thoracic spine, or thoracolumbar junction. This is the most common form. It is associated with a non-structural hyperlordosis of the lumbar and cervical spine.
- Type II: Also known as lumbar or atypical Scheuermann's disease. This is characterised by endplate changes without significant wedging and leads to loss of lumbar lordosis. It is rare and is more likely to progress during adult life.
Aetiology
Pathophysiology
The pathophysiology of type I SD is not completely understood. It starts prior to puberty after ossification of the vertebral ring apophysis and is most prominent during the adolescent growth spurt. There appears to be disconcordant vertebral endplate mineralisation and ossification which leads to disproportional vertebral body growth and wedge formation.
Pathophysiological theories include:
- Genetic: It seems to have an autosomal dominant inheritance pattern with high penetrance and variable expressivity. A large twin study that looked at self-reported previous diagnoses of SD found that there was a major genetic contribution with odds ratios of 32.92 in monozygotic and 6.25 in dizygotic twins, and heritability of 74%. [3]
- Mechanical factors: Repetitive activities including repetitive axial loading in the immature spine. It is thought that there may be an altered remodelling response to abnormal biomechanical stress. Individuals with SD tend to be heavier and taller but this may be a consequence of other upstream factors (e.g. hormonal), rather than a cause of SD itself.
- Vertebral development: There is a disturbance of endochondral ossification of the vertebral bodies and defective collagen fibril formation with increased proteoglycan levels. There is a slowed down or absence of growth in affected areas, while in the normal areas there is acceleration of growth. Increased kyphosis leads to increased pressure on the pathological bone which it can't withstand, creating a vicious cycle of increased wedging, increased kyphosis, and increased load on the vertebral bodies.[2]
- Increased growth hormone levels. Individuals with SD tend to be slightly heavier and taller.
- Juvenile osteoporosis: many studies have found lower bone mineral density in SD patients. However it isn't known if this is a consequence of decreased physical activity due to pain, or a primary causative factor of SD.
- Sternum length: There is an association of shorter sternums with SD development. It isn't known if this is a primary cause, but if it is it could be explained by the shorter sternum causing increased forces on the anterior aspect of the thoracic vertebrae.
- Trauma
- Vitamin A deficiency
- Poliomyelitis
- Epiphysitis
Type II SD is often seen in athletic adolescent males or in those that do heavy lifting as a consequence of axial overload.[2]
Sources of Pain
The source or sources of pain are not well understood. In adults, disc degeneration and facet joint osteoarthritis are potential pain generators in the affected and adjacent segments that compensate for the curvature.[2] Provocative discography is often positive in affected individuals.[4] In children, theories include paravertebral muscle fatigue, and when the primary deformity exceeds the ability of the adjacent segments to adapt to it.[2]
Epidemiology
Prevalence studies report a range of 0.4 to 8%. These figures may be a lower limit due to the condition being generally underdiagnosed. Most studies indicate a slight male predominance. It typically presents between the ages of 12 and 15.[1]
Clinical Features
History
The most common reason for individuals with SD seeking healthcare is cosmetic concern related to deformity.
In type I disease, patients may have pain, but this is usually mild, and tends to improve when growth is completed. The pain is generally located at the apex of the curve and may be worse with activity. Spondylolisthesis is more common in SD and patients with this may have low back pain. In type II SD the lumbar spine is stiff and pain is more prominent.[2]
Examination
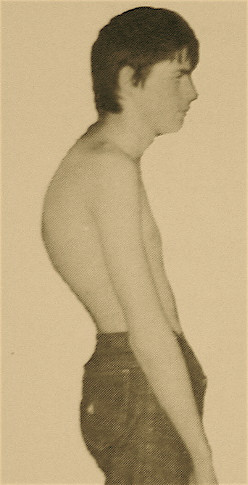
Spinal range of motion should be assessed in all planes: flexion/extension, lateral bending, and rotation. The kyphosis is present in the thoracic or thoracolumbar region and is fixed, i.e. it is still present with hyperextension of the spine. The Adam's test involves having the patient bend forward, and is positive if there is a sharply angulated deformity (a gibbus).[1]
There is a varying degree of compensatory lumbar hyperlordosis with a negative sagittal balance and forward head posture. These lumbar and cervical curves are flexible. Scoliosis is present in approximately a third of patients and tends to be minor.
There may be hyperpigmentation at the apex of the kyphosis. This is thought to be due to skin friction from sitting on chairs. There is sometimes shortening of the anterior shoulder girdle, hamstring, and iliopsoas muscles. There may be localised tenderness at the apex of the curve.
Neurological abnormalities are rare. Deficits may occur in the presence of thoracic disc herniation, kyphotic angulation, spinal cord tenting, extradural spinal cysts, osteoporotic compression fractures, and anterior spinal artery injury.
Restrictive lung disease may occur in the presence of severe angulation of over 100° where the apex of the curve is in the 1st to 8th thoracic segments.[5]
Imaging
The standing lateral radiograph is used to make the diagnosis. The arms elevated or held on the ipsilateral clavicles. The pelvis and hips are included for calculation of spinopelvic measurements.
Most studies use the 1964 Sorenson criteria:
- Thoracic Cobb angle of at least 40° or thoracolumbar kyphosis of >30°
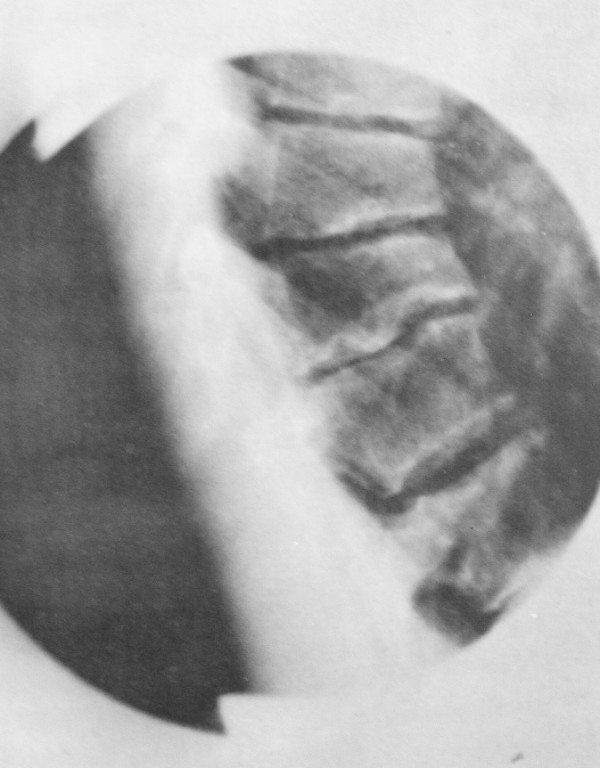
- Anterior vertebral wedging in three or more adjacent vertebra, greater or equal to 5°
The 1987 Drummond criteria only required two or more adjacent vertebrae. The 1987 Sachs criteria only require one vertebra to be wedged along with a thoracic kyphosis of more than 45° (T3-T12).[1]
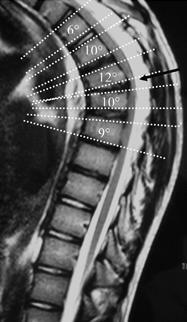
Cobb Angle Measurement on Lateral Imaging[6]
- Line is drawn along the superior endplate of the most tilted vertebrae on the cephalad portion of the kyphotic curve
- Line is drawn along the inferior endplate of the most tilted vertebrae on the caudal portion of the kyphotic curve
- The angle formed by the intersection of lines perpendicular to the above-described lines is the measured Cobb angle
- Hyperkyphosis is described as, measured Cobb angle greater than 40 degrees
Anterior Wedging Measurement on Lateral Imaging[6]
- Line is drawn from posterior to anterior along the superior endplate
- Line is drawn from posterior to anterior along the inferior endplate
- The angle formed by the intersection of these lines anteriorly is the measured Wedge angle
- Anterior wedging of greater than or equal to 5 degrees in three or more adjacent vertebral bodies, with an associated rigid hyperkyphosis greater than 40 degrees, is diagnostic for Scheuermann disease
Normal Values
- Thoracic kyphosis: 25° to 45°. It tends to increase with age in the normal population and is slightly greater in women. It increases with age from 20° in childhood, to 25° in adolescents, to 40° in adults.[7]
- Lumbar lordosis: 36° to 56°.
- Transitional T10-L2 zone: slightly lordotic at 0° to 10°.
Other signs of SD include vertebral endplate irregularities due to extensive disc invagination, and intervertebral disc space narrowing most pronounced anteriorly. Disc degeneration can occur, and generally occurs in the apex of the kyphosis. SD is associated with Schmorl's nodes, limbus vertebrae, scoliosis, and spondylolisthesis.
The sagittal balance of the spine can be assessed by looking at the C7 plumb line. This is the C7 vertebral body vertical axis and it should lie vertically within 2cm of the sacral promontory. In patients with SD the spine tends to have a negative balance with the C7 plumb line lying behind the sacral promontory.[1]
The rigidity of the kyphosis can be assessed by imaging the patient while they lie in hyperextension over a bolster.[8]
MRI is performed in some cases to assess for disc and spinal cord abnormalities.
Differential Diagnosis
The main differential is postural kyphosis. These conditions can be differentiated by the forward bending test. In postural kyphosis there is a smooth, flexible, and symmetric contour. In SD, there is an area of angulation in a fixed kyphotic curve.[1]The postural kyphotic curve is correctable in hyperextension and in supine.[8]
- Scheuermann's disease
- Postural kyphosis
- Idiopathic kyphosis
- Ankylosing spondylitis
- Osteochondral dystrophies
- Spondyloepiphyseal dysplasias
- Congenital kyphosis
- Tuberculosis
The Schmorl's nodes and endplate irregularities can be so severe that lower lumbar SD can be confused with infection, tumours, or other conditions.[9]
Treatment
Monitoring
Patients are periodically followed up by x-rays until skeletal maturity. Lumbar Scheuermann's disease is non-progressive and typically resolves with rest, activity modification, and time. [1]
Physiotherapy, Osteopathy, and Manual Therapy
Physiotherapy generally includes hamstring stretching, trunk extensor strengthening, and core stability exercises. Extension sports such as gymnastics, aerobic exercise, swimming, basketball, cycling, and hyperextension exercises are advised. In those with type II disease, sports involving functional overuse of the back are not recommended.[8][1]
Physical therapy can result in a modest reduction in pain in those with mild disease. One study reported on the outcomes of 351 patients with SD and a kyphosis of < 65° and average age of 17 to 21 years old. They used an intensive multi-modal approach with osteopathy, postural education, acupuncture, manual therapy, McKenzie, and Brugger modalities, as well as psychological intervention. There was no control group. They reported a pain reduction between 16 to 32%. [10]
Physical therapy can also be used alongside bracing to prevent spinal stiffness.[8]
There is no conclusive evidence that physical therapy on its own can result in improvement of the kyphosis.[1]
Bracing
The indications for bracing are not completely understood, it isn't known how to select those at risk of progression, nor is it known whether this treatment can improve pain. In appropriate patients, bracing can result in improvement of kyphosis and reversal of vertebral wedging. However once the brace is removed the correction is often partially lost. This results in only a modest long-term correction of the deformity, with one study showing a long-term average correction of the kyphosis of only 6° [8][1]
Patients with an apex above T7 have traditionally been given a Milwaukee brace.[1] Other brace options exist such as the Kyphologic brace which is more discrete than the Milwaukee.[11] In those with a low thoracic or thoracolumbar kyphosis are given an underarm orthosis with anterior infraclavicular outriggers.[1] Lumbar kyphosis can be treated with a physio-logic brace aimed at restoring the lumbar lordosis.[11]
The brace should be worn between 16-23 hours a day for 18 months. Subsequently it should be worn for part of each day for an additional 18 months, and then gradually withdrawn at the time of skeletal maturity.[8]
| Predictors of Favourable Outcome | Predictors of Poor Outcome |
|---|---|
|
|
The presence of scoliosis does not have an impact on the outcome.[8]
Individuals treated with a brace often have difficulties in certain activities such as sleeping and getting out of bed. There is an increased risk of low back pain with increased bracing time, especially in girls. There is often low compliance with treatment.[11]
Surgery
There is limited evidence regarding the indication for surgery nor long-term outcome following surgery. General factors considered when recommending surgery include:[8][2]
- Neurological compromise (spastic paraparesis). This is the only absolute indication.
- Progressive severe kyphosis of > 70° uncontrolled by bracing
- Disabling pain with failure of conservative management
- Genuine cosmetic concern
There are various surgical techniques which is usually done from a posterior approach with instrumentation. The description of the techniques is outside the scope of this article.[8] The available evidence indicates satisfactory outcomes in terms of pain and cosmesis.[2]
Surgery can result in significant complications. In SD, the spinal cord is shortened, and there is a risk of neurological injury as a result of acute lengthening of the cord. Therefore to avoid cord injury surgery involves simultaneous shortening of the posterior vertebral column with multi-segmental osteotomies. Other risks include lung injury (with anterior approach), infection, implant failure, non-union, junctional kyphosis, and pseudoarthrosis with recurrent deformity. Recurrent deformity can occur due to continuous bending as the bone graft is healing under tension. If junctional kyphosis occurs the fusion may need to be extended.[8]
Prognosis
The understanding of the long-term prognosis is limited, as is the risk of progression at any given degree of angulation. In most cases it is thought to have a benign self-limiting course,[8] with no major interference in life in the long-term.
One study compared 67 adults with SD who were managed non-operatively with a mean kyphosis of 72° and mean follow-up of 32 years to a control group. The SD group had higher pain intensity, more frequent thoracic pain, had jobs that required less activity, and had weaker trunk extension with reduced range of motion of extension. 38% of SD patients had severe interference of pain with daily living compared to 21% in the control group but this wasn't statistically significant. There was no significant difference in sick leave due to back pain, analgesic use for back pain, or participation in recreational activities.[5]
Another study looked at 49 patients with SD who were managed non-operatively after a mean follow up period of 37 years and compared them to a control group. Patients with SD had a fourfold higher risk for back pain during the past 30 days, and threefold higher risk for continuing back pain compared to controls. 16% had pain in the thoracic spine, 31% in the lumbar spine, 29% in the whole spine, and 25% had no back pain. Patients with SD had a higher risk for back pain related disability compared to controls. There was no association with kyphosis severity and disability. Certain activities of daily living were more difficult in those with SD such as carrying a 5-kg load at least 100m, and walking up one floor without resting. There was no difference in absences from work due to back pain.[12]
Disc degeneration occurs mainly at the apex of the kyphosis and can lead to pressure on the cord with increasing age. There is an increased risk of neural complications with a sharply angulated, short-segmented curve. [8]
Summary
- SD is generally a benign self-limiting condition but can lead to a higher risk for back pain in adulthood.
- Severe kyphosis over 70° can cause more severe problems in adulthood with back pain, restrictive lung disease, and neurological complications.
- Thoracic kyphosis is measured from the most proximal to most distal vertebra included in the curve using the Cobb method.
- Bracing has a limited role in mild disease in growing patients but the deformity can recur after cessation of treatment
- The indications and long-term outcomes of surgery are unknown, and it is associated with a significant risk of complications.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Bezalel et al.. Scheuermann's disease: current diagnosis and treatment approach. Journal of back and musculoskeletal rehabilitation 2014. 27:383-90. PMID: 24898440. DOI.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Boos, Norbert, and M Aebi. Spinal disorders : fundamentals of diagnosis and treatment. Berlin New York: Springer, 2008.
- ↑ Damborg et al.. Prevalence, concordance, and heritability of Scheuermann kyphosis based on a study of twins. The Journal of bone and joint surgery. American volume 2006. 88:2133-6. PMID: 17015588. DOI.
- ↑ Singh V, Manchikanti L, Shah RV, Dunbar EE, Glaser SE. Systematic review of thoracic discography as a diagnostic test for chronic spinal pain. Pain Physician. 2008 Sep-Oct;11(5):631-42. PMID: 18850027.
- ↑ 5.0 5.1 Murray PM, Weinstein SL, Spratt KF. The natural history and long-term follow-up of Scheuermann kyphosis. J Bone Joint Surg Am. 1993 Feb;75(2):236-48. doi: 10.2106/00004623-199302000-00011. PMID: 8423184.
- ↑ 6.0 6.1 Mansfield JT, Bennett M. Scheuermann Disease. [Updated 2020 Aug 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499966/
- ↑ Fon et al.. Thoracic kyphosis: range in normal subjects. AJR. American journal of roentgenology 1980. 134:979-83. PMID: 6768276. DOI.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 Tsirikos & Jain. Scheuermann's kyphosis; current controversies. The Journal of bone and joint surgery. British volume 2011. 93:857-64. PMID: 21705553. DOI.
- ↑ Wenger & Frick. Scheuermann kyphosis. Spine 1999. 24:2630-9. PMID: 10635526. DOI.
- ↑ Weiss et al.. Effect of intensive rehabilitation on pain in patients with Scheuermann's disease. Studies in health technology and informatics 2002. 88:254-7. PMID: 15456045.
- ↑ 11.0 11.1 11.2 Weiss, Hans-Rudolf et al. “Brace treatment for patients with Scheuermann's disease - a review of the literature and first experiences with a new brace design.” Scoliosis vol. 4 22. 29 Sep. 2009, doi:10.1186/1748-7161-4-22
- ↑ Ristolainen L, Kettunen JA, Heliövaara M, Kujala UM, Heinonen A, Schlenzka D. Untreated Scheuermann's disease: a 37-year follow-up study. Eur Spine J. 2012 May;21(5):819-24. doi: 10.1007/s00586-011-2075-0. Epub 2011 Nov 22. PMID: 22101868; PMCID: PMC3337904.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,