TMJ Injection
From WikiMSK
| TMJ Injection | |
|---|---|
| Indication | |
| Syringe | 1mL |
| Needle | 27-gauge 3.2 mm |
| Injectate | steroid, dextrose, or PRP |
| Steroid | 20mg triamcinolone |
| Volume | 1mL |
Anatomy
Indications
Contraindications
Pre-procedural Evaluation
Equipment
Technique
Landmark Guided
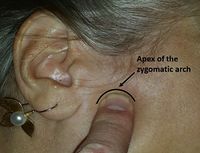
Injection entry site, located 0.75 to 1 cm below the apex of the zygomatic arch.[1]

Injection angle of 45° cranial and 10° posterior, using a 1-in 30-G needle.[2]
- Relaxed, closed-mouth approach.
- Injector’s index finger is placed in the depression under the zygomatic arch, against the zygoma, and draw a curved line approximating the bottom of the arch.
- Ask the patient to open and close their mouth and confirm the location of the posterior mandible. Feel the head of the mandible pass anteriorly underneath your finger and then resume its posterior position
- Needle (27-gauge 3.2 mm) entry is 1cm below the apex of the zygomatic arch with slight (<15 degree) posterior angulation, and 45 degree of cephalad angulation
- Inject 1 mL was at 25 mm depth
- If the needle inadvertently made bony contact shallower than 25 mm, withdraw the needle several mm and redirect superiorly to avoid the mandibular condyle.
Ultrasound Guided
- Challenging, consider using if failed landmark guided injection
- Use a high-frequency linear small footprint probe
- Place longitudinally over the TMJ.
- Visualise superficial temporal vessels overlying the joint.
- Move probe cranially to allow needle to enter skin
- Enter joint space in plane with 23G needle
- Inject solution into joint space
Complications
damage to the collateral ligaments of the disc and the adjacent soft tissue
Aftercare
Avoid NSAIDs if prolotherapy or PRP.
Videos
See Also
External Links
References
- ↑ Louw et al.. Treatment of Temporomandibular Dysfunction With Hypertonic Dextrose Injection (Prolotherapy): A Randomized Controlled Trial With Long-term Partial Crossover. Mayo Clinic proceedings 2019. 94:820-832. PMID: 30878157. DOI.
- ↑ Louw et al.. Treatment of Temporomandibular Dysfunction With Hypertonic Dextrose Injection (Prolotherapy): A Randomized Controlled Trial With Long-term Partial Crossover. Mayo Clinic proceedings 2019. 94:820-832. PMID: 30878157. DOI.
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,


