Tendinopathy
Pathology
There are profound changes in tendon structure in tendinopathy. In tendinopathy there is loss in capacity to take load through an area of degeneration. This leads to difficulty in stimulating any reparative response.
It isn't possible to tear normal collagen. With tendon rupture there is rupture of highly degenerative tendon without much normal tissue. Therefore without a collagen tearing response, there is no bleeding response, and without bleeding there is no inflammatory response.
inflammation occurs in tendinopathy, however it is low grade. There are a few inflammatory cells peri-vascularly, and also some inflammatory mediators that are possibly produced by tenocytes and visiting cells. Inflammation is unlikely to be the prime driver of pathology or pain, and in the past when management was directed at this, outcomes were poor. While with rupture or bleeding in a tendon, inflammation is a prime driver.
The tendon cells, called tenocytes, are intimately connected to collagen and are sensitive and responsive to matrix load. The mechano-set point of the cell is dependent on the load that is placed on it, and this can be up- or down-regulated, with positive and negative responses.
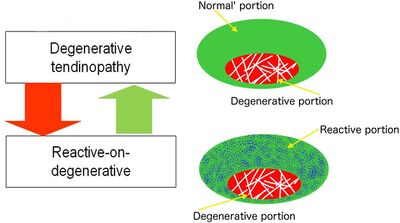
Degenerative tendinopathy is a terminal state and cannot be recovered. In early stages of tendon pathology it is reversible, however once the tissue is in a non-load bearing state, there is no capacity to repair. Tendon healing and repair requires load sensitivity and a normal cell. In a degenerative tendon there is no collagen structure, and the pathology is therefore mechanically "deaf" and receives no stimulus to repair. The cells are also chondrocytic, they undergo metaplasia from a tenocyte to a chondrocyte, and produce a lot more cartilage based proteoglycans and type III as well as type II collagen. Therefore the normal structure cannot be recovered. However tendon pathology is not passive, it has a metabolic rate 25 times higher than a normal tendon, but it has no stimulus to create structure, and so this is all in vain.
A tendon with degeneration will also have an area of normal tendon. So with rehabilitation when there is overload placed only on the normal tendon, a reactive change occurs in the normal tendon. This is called reactive on degenerative tendinopathy. The normal tendon around a degenerative area protects the tendon by structuring more normal tissue. Most individuals with degeneration form enough normal tendon to allow the structure to take load, in fact usually even more than a non-degenerative tendon. This relates to the management principle of "treat the doughnut not the hole." It also counters the theory that regenerative procedures such as platelet rich plasma injections are needed, as the body has already done this. We cannot identify the tendons that are at risk of rupture or poor outcomes either from imaging or clinical presentation.
Tendon pain only occurs in the presence of pathology. Pathology increases the risk of pain 3-7 times, however pathology can occur without pain. The nociceptive driver is unknown, but it may be the cell, and not due to inflammation or neovascularisation. The sensory innervation of the tendon is quite peripheral, in the peritendon, muscle, and bone-tendon junctions. There is little to no innervation within the tendon due to the massive loads placed on the tendon, which would theoretically cause pain even with a normal structure.
There are motor and sensory changes consistent with nociception, with changes in excitability and inhabitation to the affected muscle of the painful tendon. The changes all relate to motor-drive, and there is no evidence of any central or nociplastic drive to pain.
Clinical Features
Tendon pain is local, aggravated by load, and eased by unloading.
Treatment
Active Therapies
Exercise
Improvements in pain and function due to exercise are not linked to structural changes. Effective exercise acts on the normal part of the tendon, increasing its tolerance to load. Exercise also acts on the muscles, the kinetic chain, and the brain.
Passive Therapies
Passive therapies should be an adjunct, and have to sit within the context of the load capacity and load tolerance of the tendon, muscle, kinetic chain, and brain. Passive therapy alone cannot result in improvement without an active intervention as well.
Injection therapies
Most injection therapies have a neural effect. Sclerosing injections are neurotoxic, and may be causing pain relief due to possible cytotoxic effects on the nociceptors. It likely doesn't matter what is injected, even saline has an effect, and so the effect of any injection is likely not acting on the pathology, but rather the neural drive.
PRP injection results in increased cell and GAG content, however this forces the tendon further away from normal, and there is no increase in collagen count. The tendon is already hypercellular and producing a large amount of GAGs. Needle tracts can be seen even months later on UTC scan, and so even the physical act of needle insertion into a tendon negatively affects tendon structure.
Shockwave therapy
Pain relief may be due to neuropraxic effects on the nerves. However shockwave therapy can actually negatively affect the structure for up to 6 weeks.
Rest
Passive therapies such as injections come with prescribed rest, and so at least part of the beneficial effect on pain will be due to reducing load. Subsequently after resting when initiating load again the pain is worse due to the rest.
Surgery
Extra-tendinous surgery denervates the tendon, leading to pain relief, but not improving the load tolerance.
Intra-tendinous surgery results in an inflammatory proliferative response which leads to some type I collagen production but it doesn't result in pain relief.
Further Reading
- Open access article on the continuum model of tendon pathology by Cook et al.[1]

