Tendinopathy
Pathology
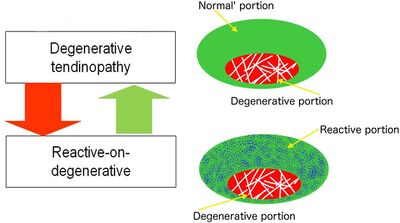
There are profound changes in tendon structure in tendinopathy. In tendinopathy there is loss in capacity to take load through an area of degeneration. This leads to difficulty in stimulating any reparative response.
It isn't possible to tear normal collagen. With tendon rupture there is rupture of highly degenerative tendon without much normal tissue. Therefore without a collagen tearing response, there is no bleeding response, and without bleeding there is no inflammatory response.
The tendon cells, called tenocytes, are intimately connected to collagen and are sensitive and responsive to matrix load. The mechano-set point of the cell is dependent on the load that is placed on it, and this can be up- or down-regulated, with positive and negative responses.
Inflammation
inflammation occurs in tendinopathy, however it is low grade. There are a few inflammatory cells peri-vascularly, and also some inflammatory mediators that are possibly produced by tenocytes and visiting cells. Inflammation is unlikely to be the prime driver of pathology or pain, and in the past when management was directed at this, outcomes were poor. While with rupture or bleeding in a tendon, inflammation is a prime driver.
Terminal state
Degenerative tendinopathy is a terminal state and cannot be recovered. In early stages of tendon pathology it is reversible, however once the tissue is in a non-load bearing state, there is no capacity to repair. Tendon healing and repair requires load sensitivity and a normal cell. In a degenerative tendon there is no collagen structure, and the pathology is therefore mechanically "deaf" and receives no stimulus to repair. The cells are also chondrocytic, they undergo metaplasia from a tenocyte to a chondrocyte, and produce a lot more cartilage based proteoglycans and type III as well as type II collagen. Therefore the normal structure cannot be recovered. However tendon pathology is not passive, it has a metabolic rate 25 times higher than a normal tendon, but it has no stimulus to create structure, and so this is all in vain.
Reactive change
A tendon with degeneration will also have an area of normal tendon. So with rehabilitation when there is overload placed only on the normal tendon, a reactive change occurs in the normal tendon. This is called reactive on degenerative tendinopathy. The normal tendon around a degenerative area protects the tendon by structuring more normal tissue. Most individuals with degeneration form enough normal tendon to allow the structure to take load, in fact usually even more than a non-degenerative tendon. This relates to the management principle of "treat the doughnut not the hole." It also counters the theory that regenerative procedures such as platelet rich plasma injections are needed, as the body has already done this.
Tendon pain
Tendon pain only occurs in the presence of pathology. Pathology increases the risk of pain 3-7 times, however pathology can occur without pain. The nociceptive driver is unknown, but it may be the cell, and not due to inflammation or neovascularisation. The sensory innervation of the tendon is quite peripheral, in the peritendon, muscle, and bone-tendon junctions. There is little to no innervation within the tendon due to the massive loads placed on the tendon, which would theoretically cause pain even with a normal structure.
There are motor and sensory changes consistent with nociception, with changes in excitability and inhabitation to the affected muscle of the painful tendon. The changes all relate to motor-drive, and there is no evidence of any central or nociplastic drive to pain.
Pain, poor function, and pathology/poor structure are three different concepts that often overlap, but can also exist on their own.
Biomechanics
Key tendon functions
- Energy storage and release. Tendons are viscoelastic. With slow stretching the tendon gets longer and doesn't store energy. In order to store energy on the tendon the tensile loading needs to be fast.
- Compression. Compression occurs just proximal to the tendon insertion. For example with the achilles tendon, there is compression of the tendon against the back of the calcaneum with dorsiflexion. Any hagland 'deformity' actually reduces the load on this tendon.
- Friction. This where the tendon moves too much through the peritendon structures, leading to a peritendon response.
There is only one true tendinopathy in the lower limb. The rest are enthesopathies and are prone to compressive loads, the best representation of that is gluteus medius/minimus tendon pain.
Clinical Features
Tendon pain is local, aggravated by load, and eased by unloading. Tendon pain can completely destroy the kinetic chain, and so in that way it can be very different to pain in other structures.
Pain is directly linked to overload. Overload can be a one off event, leading to an acute tendon tendon response - either reactive or reactive on degenerative. It will usually settle quickly if its only a single load.
With ongoing tendon pain, there is often a drive to stop loading the tendon, leading to negative consequences with loss of power, strength, and endurance. The reduced capacity means high load on the tendon with less activity, and therefore more pain.
Part of the clinical assessment is around working out which loads are clinically relevant - energy storage and release, compression, and/or friction.
Diagnosis is based on imaging abnormality and palpation tenderness, however neither of these are diagnostic of tendon pain.
In the setting of tendon rupture, two thirds never had pain.
Differentiating Lower Limb Tendinopathy
Most tendons have a clear clinical profile such as the patellar and gluteus medius tendons, while the achilles tendon is harder to fit into a neat category. Athletes present with true tendinopathies, as tendinopathy is a condition of power athletes. Up to 40% of athletes have pathology but only a small number have symptoms. To some extent tendinopathy is a condition of older age but this is due to load exposure throughout life rather than age itself, and so highly active young athletes can have tendinopathy.
Patellar tendinopathy - young men, jumping sport, occasionally in elite female athletes but half the rate as men. There is a central cyclops lesion. This is over-diagnosed as a cause of anterior knee pain, and the correct diagnosis is usually patellofemoral pain syndrome. Patellofemoral pain can generate pain over the tendon, and so pain inferior to the patellofemoral joint can still be patellofemoral pain.
Gluteal tendinopathy - post-menopausal women, very occasionally in men. There is compression in women not men due to the broader pelvis in women. It is rare to find an intact tendon in women over the age of 60.
Adductor tendinopathy - This is difficult to diagnose due to multiple sources of pain. There is no cortical bone between bone and tendon. More common in men.
Achilles tendinopathy - non-homogenous presentation. There is loading across life, with simple everyday tasks being energy storage tasks such as walking up and down stairs. Therefore relatively sedentary people can present with this. There may also be an endurance capacity issue.
There can be a combination of pain sources, with tendon pain being only part of the problem. Overall it does tend to be mostly the tendon or not at all. In the patellar tendon we don't see a mix of nociceptive input.
Tenderness is a poor diagnostic sign. Tenderness relates to how much the tendon has been used, and isn't linked to either symptoms or imaging findings. Tenderness is likely to persist even with recovery in pain and function, and so don't allow the patient to poke it as it isn't an indicator of progress. The patellar tendon is the most painful area to palpation in the knee in both osteoarthritis and patellofemoral joint pain. This doesn't correlate with pain provocation testing (decline squat), or imaging.
Imaging
Imaging cannot diagnose the source of the problem, and also does not assist in predicting the prognosis. Imaging can also lead to harms as terms like tear and degeneration can result in kinesiophobia.
Much of what is seen on imaging is subjective with poor interobserver reliability. This includes amount of disorganisation, doppler flow, and intratendinous tears. In ultrasound an MR imaging of gluteal tendon pathology, using surgical/histological findings as the gold standard, there is good-to-excellent reliability for ultrasound in diagnosing full-thickness tears. However there is extremely poor reliability for tendinosis and partial tears for both MRI and US.[2]
A normal scan is highly predictive of the pain not coming from that tendon. However half of 'normal' tendons with pain develop pathology on imaging over one year. Abnormal imaging only means that the pain might be coming from the tendon, not that it is coming from there. There is a 3-18 times increase odds of having pain with an abnormal tendon on imaging.
Vascularity is not a cause of pain, but simply a marker of tendon degeneration.
We cannot identify the tendons that are at risk of rupture or poor outcomes either from imaging or clinical presentation.
The language used is very important. Explain to patients that they should not worry about the tear. This is because of the poor inter-observer reliability and that it rarely affects treatment. Also explain that a thick tendon is a good tendon, and shows that the tendon has compensated, and there is enough "doughnut" around the hole. The tendon isn't limiting them becoming pain-free but rather dysfunction is the problem.
Management
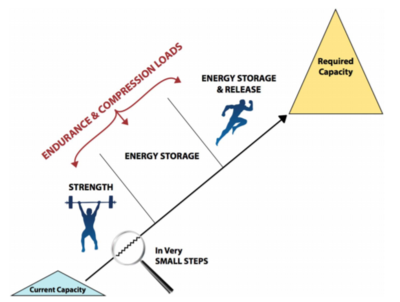
Management Approach Overview
- Ensure the tendon is the source of the pain. Imaging changes do not mean that the tendon is causing the pain. The wrong diagnosis will lead to the wrong treatment.
- Assess the existing muscle and tendon capacity and function
- Establish what the patient wants to achieve and the capacity and function required (e.g. sprinting athlete doesn't need endurance)
- Slowly build from one thing to the next, in very small steps. Improvement takes a long time and can't be rushed. Progress when there is stability in symptoms, and no flare with load.
- Initially unload the tendon from the tendon load that started to problem in order to control the pain and to get the correct loading on it.
- Use isometric exercises to reduce pain
- Address co-morbidities
- Use adjuncts as required.
Stage 1: Isometrics
Stage 2: Isotonics/heavy slow resistance
Stage 3: Energy Storage
Stage 4: Energy storage and release
Someone who understands tendons must set the program. Errors include incorrect or too little progressions, insufficient load (the exercise must be set at maximal tolerable load. If the load is too light then it is inefficient, if it is too heavy then you get increasing symptoms), too little attention to the kinetic chain, and following recipe programmes without addressing the individual.
An example is gluteal tendinopathy in a middle aged woman. Their current capacity is poor strength is all leg muscles, muscle wasting, poor endurance, and pain with simple loads. The required capacity is to be able to walk, shop, garden, and endurance. These patients won't be taken to energy storage and release loads. Rehabilitation will be focused on strength, usually with home-exercises, sit to stand, calf raises on two legs, isometric exercises etc. Commonly gluteal tendinopathy patients will be prescribed clams, but the load with clams is much too low and have virtually no effect.
Active Therapies
General
The general principles are exercise and correct loading. The goal of therapy is restoration of full pain-free function. In athletes there is the additional requirement of having the tendon be able to tolerate the highest load with energy storage and release. We also want the tendon to be able to tolerate loads other than tensile, mainly compression, and sometimes friction. The answer to improving pain is not changing structure.
Active therapy modalities are less likely to help when done in isolation.
Improvements in pain and function due to exercise are not linked to structural changes. Effective exercise acts on the normal part of the tendon, increasing its tolerance to load. Exercise also acts on the muscles, the kinetic chain, and the brain.
In an athlete, rehabilitation is not only about getting the tendon strong, but also getting it to a state where it can take on a high rate of tensile loading. Athletes with poor function will always have pain, and athletes with good function are protected from pain. There treatment is aimed at improving function, in order to lead to improvement in pain.
A big problem in the research in this area is that surrogate outcomes are usually used - a decrease in pain rather than looking at return to activity.
Active therapies often fail in the real world because it isn't perceived at cutting edge, it is slow, and it isn't expensive.
Isometric exercise
Isometric exercise acts on cortical inhibition, and there is relief for several hours if done correctly. It is more effective in the big muscle groups such as achilles, hamstrings, patella. Athletes are 20% stronger after isometrics. Isometric exercise also conditions the tendon.
It needs to be done with a long hold. 45 seconds for 5 repetitions, 1-2 times a day, with 2 minute rest between.
Eccentric exercise
Eccentric exercise loads the series elastic component of the tendon and muscle, i.e the spring. It doesn't strengthen the muscle, kinetic chain, change the brain, or address the individual. It doesn't adapt the tendon to energy storage loads. And it doesn't change compressive loads. The evidence is mixed and can be ineffective on their own, especially in athletes. Overall these should not be done in isolation, and not follow a set program.
Heavy slow resistance
This improves muscle strength, and the slow loads improve the mechanical strength of the tendon. Muscle endurance can be addressed with endurance sets. It doesn't fully address the kinetic chain and brain, and doesn't adapt the tendon to energy storage and release loads.
The weight needs to be heavy, but heavy for that specific person. It doesn't have to be using a squat rack. This is best done single leg, with each log. Once strength is obtained then can move on to adding function strength/endurance and energy storage and release.
Energy storage and release loading
Here you are adding speed by using plyometric exercises. This improves the loading rate capacity of the tendon. These are done 2-3 times a week only. Progress to sport based activities.
Load modification
Reduce the high loads placed on the painful tendon. For example sprinting and direction changing in an athlete. Tendon pain reduces athletic function as they can't jump, change direction, etc.
Reducing compression
In certain conditions the compressive load on the tendon should be reduced, for example with gluteus medius/minimus tendon pain.
Visual/auditory cues
Cues can improve cortical plasticity. An example is using a metronome.
Passive Therapies
Passive therapies should be an adjunct, and have to sit within the context of the load capacity and load tolerance of the tendon, muscle, kinetic chain, and brain. Passive therapy alone cannot result in improvement without an active intervention as well.
Injection therapies
Most injection therapies have a neural effect. Sclerosing injections are neurotoxic, and may be causing pain relief due to possible cytotoxic effects on the nociceptors. It likely doesn't matter what is injected, even saline has an effect, and so the effect of any injection is likely not acting on the pathology, but rather the neural drive.
PRP injection results in increased cell and GAG content, however this forces the tendon further away from normal, and there is no increase in collagen count. The tendon is already hypercellular and producing a large amount of GAGs. Needle tracts can be seen even months later on UTC scan, and so even the physical act of needle insertion into a tendon negatively affects tendon structure.
Shockwave therapy
Pain relief may be due to neuropraxic effects on the nerves. However shockwave therapy can actually negatively affect the structure for up to 6 weeks.
Rest
Rest can improve pain in the short term, but the long term consequences are profound. Rest is catabolic to the tendon. The tendon tissue loses structure and becomes pathological, and the mechanical strength of the tendon decreases in two weeks. There are also negative changes in the anti-gravity kinetic chain function and cortical motor drive. Subsequently after resting when initiating load again the pain is worse due to the rest. The strength of the tissue is only as great as the load placed on it. By decreasing load, you are decreasing tissue capacity.
Passive therapies such as injections come with prescribed rest, and so at least part of the beneficial effect on pain will be due to reducing load.
Surgery
Extra-tendinous surgery denervates the tendon, leading to pain relief, but not improving the load tolerance.
Intra-tendinous surgery results in an inflammatory proliferative response which leads to some type I collagen production but it doesn't result in pain relief.
Further Reading
- Open access article on the continuum model of tendon pathology by Cook et al.[1]
References
Article based off lecture series by Jill Cook at NZAMM conference 2020
- ↑ 1.0 1.1 Cook et al.. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research?. British journal of sports medicine 2016. 50:1187-91. PMID: 27127294. DOI. Full Text.
- ↑ Docking SI, Cook J, Chen S, Scarvell J, Cormick W, Smith P, Fearon A. Identification and differentiation of gluteus medius tendon pathology using ultrasound and magnetic resonance imaging. Musculoskelet Sci Pract. 2019 Jun;41:1-5. doi: 10.1016/j.msksp.2019.01.011. Epub 2019 Feb 7. PMID: 30763889.
- ↑ Cook & Docking. "Rehabilitation will increase the 'capacity' of your …insert musculoskeletal tissue here…." Defining 'tissue capacity': a core concept for clinicians. British journal of sports medicine 2015. 49:1484-5. PMID: 26255142. DOI.

