Corticosteroids
Injectable corticosteroids are synthetic analogues of the adrenal glucocorticoid cortisol (hydrocortisone). Cortisol is produced by the zona reticularis of the adrenal cortex. Corticosteroids have an effect at the cellular level, with modulation of the transcription of a large number of genes. They act directly on nuclear steroid receptors. They reduce the production of a variety of pro-inflammatory mediators and increase the production of anti-inflammatory mediators.
Terminology
Corticosteroids are a class of steroid hormones that are released by the adrenal cortex. The class includes glucocorticoids and mineralocorticoids. However in general use, corticosteroids generally refers to glucocorticoids.
Synthesis
The hypothalamic-pituitary-adrenal axis (HPA) controls the release of glucocorticoids. Corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) released from the hypothalamus act on the anterior pituitary to stimulate the secretion of adrenocorticotrophin hormone (ACTH). ACTH then stimulates the adrenal cortex to release glucocorticoids. As well as this feed-forward mechanism, there are also negative feedback mechanisms whereby glucocorticoids inhibit the release of ACTH and CRH.
Glucocorticoids are synthesised rapidly following ACTH stimulation because they can't be pre-synthesised and stored in the adrenal glands. They are synthesised from cholesterol in a process called steroidogenesis.
Glucocorticoids are released by the adrenal glands with a circadian profile. This circadian profile is upregulated by the hypothalamic-pituitary-adrenal axis (HPA). Cortisol levels peak in the mornings and are lowest at night.
While the systemic level of glucocorticoids is maintained by synthesis in the adrenal glands, the tissue and cellular availability is maintained through various mechanisms
- Ample protein binding which results in 95% of glucocorticoids being in an inactive form in the normal state. 80-90% of circulating glucocorticoids are bound to corticosteroid binding globulin (CBG), and 5-15% are bound to albumin. Therefore the accessibility of cortisol is regulated by the level of CBG.
- At the cellular level the tissue-specific metabolic enzyme 11β-hydroxysteroid dehydrogenase 2 (11β-HSD 2) rapidly inactivates glucocorticoids by converting cortisol to cortisone. In contrast, 11β-HSD 1 converts the inactive precursor cortisone to bioactive cortisol. In this manner, cortisone acts as a reservoir.
Most synthetic glucocorticoids do not bind to CBD and are not metabolised by 11β-HSD 2.
Molecular Structure
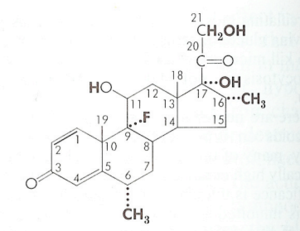
The first corticosteroid used for its anti-inflammatory effect was Cortisone. The structure was modified leading to increases in the ratio of anti-inflammatory to sodium-retaining potency. In a number of currently available compounds, the electrolyte effects are now of no serious consequence, even at the highest doses used. In all compounds the effects on inflammation and metabolism of carbohydrates and protein have paralleled one another. The effects on inflammation and metabolism are likely mediated by the same type of receptor.
Changes in the molecular structure can cause changes in biological potency. This can be due to alterations in absorption, protein binding, rate of metabolic transformation, rate of excretion, ability to traverse membranes, and intrinsic effectiveness of the molecule at its site of action. Modifications of the pregnane nucleus has been used for therapeutic value.
Ring A. The 4,5 double bond and the 3-keton are both necessary for typical adrenocorticosteroid activity. Prednisone and prednisolone introduce a 1,2 double bond. This enhances the carbohydrate-regulating potency resulting in an enhanced ratio with respect to its sodium-retaining potency. Prednisolone is also metabolised more slowly than cortisol.
Ring B. 6α substitution has unpredictable effects. For cortisol, 6α methylation increases its anti-inflammatory, nitrogen-wasting, and sodium-retaining effects. While 6α-methylprednisolone has a slightly greater anti-inflammatory potency and less electrolyte-regulating potency compared to prednisolone. Fluorination in the 9α position enhances all biological activities of the corticosteroids, apparently due to electron-withdrawing effect on the 11-beta-hydroxy group.
Ring C. Oxygen function at C 11 is indispensable for its significant anti-inflammatory and carbohydrate-regulating potency (cortisol vs 11-dexoxycortisol). However this is not necessary for its high sodium-retaining potency (desoxycorticosterone)
Ring D. 16-Methylation or hydroxylation eliminates the sodium-retaining effect but only slightly modifies potency on metabolism and inflammation
All currently used anti-inflammatory steroids are 17α-hydroxy compounds. 17-desoxy compounds (cortisol vs corticosterone) may have some carbohydrate-regulating and anti-inflammatory effects but full action requires the 17α-hydroxy substituent. All natural corticosteroids and most of the active synthetic analogues have a 21-hydroxy group. This is required for significant sodium-retaining activity, but some glycogenic and anti-inflammatory effects may occur without it.
Metabolism
Cortisol metabolism has been studied extensively. It is generally assumed that other related compounds have similar metabolism. Cortisol has a plasma half-life of around 1.5 hours. The metabolism and half-life of corticosteroids is greatly slowed down with the presence of a 1,2 double bond or a fluorine atom. Laboratories measure urinary cortisol and metabolites with the reduced ring A as "17-hydroxycorticosteroids." These compounds and those where the ketone at carbon 20 has been reduced are included in the group "17-ketogenic steroids." Urinary metabolites that have lost their side chain are included in the "17-ketosteroids." It is estimated that the liver metabolises at least 70% of secreted cortisol.
Dose Equivalency
| Glucocorticoid | Approximate Equivalent Dose (mg) |
|---|---|
| Cortisol | 20 |
| Cortisone | 25 |
| Hydrocortisone | 20 |
| Prednisone | 5 |
| Prednisolone | 5 |
| Methylprednisolone | 4 |
| Triamcinolone | 4 |
| Betamethasone | 0.6-0.75 |
| Dexamethasone | 0.75 |
Mechanism of Action
Injected corticosteroids are thought to suppress pro-inflammatory mediators and upregulating anti-inflammatory mediators. There may be a direct chondroprotective effect on cartilage metabolism, for example it promotes the production of articular surfactant. There may also be a direct analgesic effect.
Various components of the inflammatory response are suppressed through direct and indirect modulation of the transcript of certain target genes, resulting in a reduction in the levels of pro-inflammatory mediators. There are also some anti-inflammatory proteins that are upregulated.
Annexin A1 (also known as lipocortin 1) is an important endogenous glucocorticoid regulated protein that contributes to resolving inflammation by various mechanisms. It reduces neutrophil recruitment and the production of pro-inflammatory mediators, and promotes the clearance of apoptotic cells by macrophages. Annexin A1 deficient mice are resistant to glucocorticoid treatment and have a stronger and more prolonged inflammatory reaction. An ongoing research based question is whether Annexin A1 based pharmacological interventions could be as effective as exogenous glucocorticoids with fewer side effects.[2]
Glucocorticoids exert their action through a diverse collection of glucocorticoid receptors.
Choice for Injection
There is limited evidence to help guide the choice of a specific glucocorticoid for injection. Depot formulations stay at the injection site displaying mostly local effects, but some system effect can happen. The choice is largely based on availability, cost, and versatility. At the time of writing, the funded corticosteroids for depot injections are dexamethasone, methylprednisolone, and triamcinolone. Betamethasone is also available but is only partially funded. Dexamethasone is available on medical practitioner supply order, but only 5 vials at a time. Many practitioners tend to use particulate corticosteroids (like triamcinolone) for joint and soft tissue injections, and non-particulate corticosteroids (like dexamethasone) injections where there is a rare risk of serious vascular injury like spinal injections.
Side Effects
Injection therapy is relatively safe. Side-effects include post-injection flare of pain, subcutaneous atrophy and/or skin depigmentation, bleeding, steroid 'chalk' or 'paste' found in surgery, soft-tissue calcification, steroid arthropathy, tendon rupture and atrophy, delayed soft tissue healing, infection, nerve damage, transient paresis, needle fracture, facial flushing, increased systemic glucose levels, uterine bleeding, suppression of the hypothalamic-pituitary axis, allergic reaction, pancreatitis, nausea, dysphoria, acute psychosis, myopathy, posterior subcapsular cataracts.
Further Reading
- Open access review article: Ramamoorthy, Sivapriya, and John A Cidlowski. “Corticosteroids: Mechanisms of Action in Health and Disease.” Rheumatic diseases clinics of North America vol. 42,1 (2016): 15-31, vii. doi:10.1016/j.rdc.2015.08.002 Full Text
References
- ↑ Liddle 1961, Clinical Pharmacology and Therapeutics
- ↑ Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J Immunol Res. 2016;2016:8239258. doi: 10.1155/2016/8239258. Epub 2016 Jan 13. PMID: 26885535; PMCID: PMC4738713.

