Local Anaesthetics
Local anaesthetics are unique in their ability to temporarily block conduction along nerves. They interrupt nerve conduction through the inhibition of influx of sodium ions, and 90% of sodium channels must be blocked in order to get conduction blockade.
Chemistry
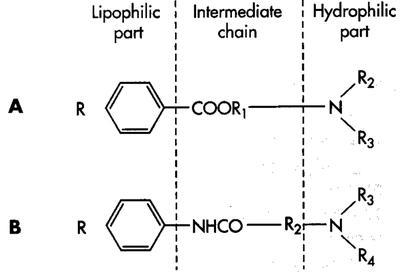
Local Anaesthetic molecules have three components: the lipophilic aromatic ring, the intermediate ester or amide chain, and the terminal hydrophilic amine.
| Esters | Amides |
|---|---|
|
|
Pharmacology
There are four principal characteristics of local anaesthetics.
| Characteristic | Correlate | Mechanism |
|---|---|---|
| Lipid solubility | Potency | Increased lipid solubility increases diffusion into neurons. |
| pKa | Time of onset | Lower pKa agents have a greater proportion of molecules in the lipid-soluble state, decreasing time of onset |
| Chemical linkage | Metabolism | Esters are primarily hydrolysed by cholinesterases in plasma. Amides are primarily metabolised in the liver |
| Protein binding | Duration | Increased protein binding at the receptor site prolongs the duration of action |
In solution, weak acids and weak bases are present in some combination of ionised and non-ionised forms. The non-ionised forms are lipid soluble, while the ionised forms are water soluble. Local anaesthetics are weak bases. The aromatic ring determines the degree of lipid solubility of the local anaesthetic. The terminal amine acts as an "on-off switch." It can exist in either a lipid-soluble tertiary non-ionised form (3 bonds) or a positively-charged ionised water-soluble quaternary form (4 bonds). Increasing lipid solubility results in a non-linear increase in potency. The relationship between lipid solubility, pH (of the tissue), and pKa (of the drug) is called the "pH-partition theory," and the proportion of ionised to non-ionised forms is called the lipid-water partition coefficient. Note, this relationship doesn't explain the lipid solubility of all drugs such as fluoroquinolones but that is another topic.[1] The pKa is the pH at which 50% of the molecules are in the non-ionised active form and 50% in the ionised inactive form.
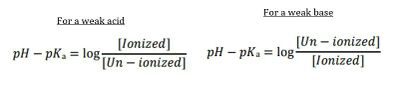
The relationship can be estimated using the Henderson-Hasselbalch equation by looking at the ratio of non-ionised (B for base) to ionised molecules (BH+ for base a proton) as per figure 2. The pH is usually assumed to be 7.4 which is the pH of extracellular fluid in normal non-infected states. The pKa varies depending on the drug.
Weak bases are more lipid-soluble in an alkaline solution, and more water soluble in an acidic solution. Conversely, weak acids are more lipid-soluble in an acidic solution, and more water soluble in an alkaline solution. Mnemonic: BASES ionise BELOW their pKa, and ACIDS ionise ABOVE their pKA
Agents with a higher pKA have more molecules in the ionised form at a pH of 7.4. Conversely agents with low pKAs have more molecules in the non-ionised form at a pH of 7.4, and therefore will have a quicker onset of action.
All local anaesthetics have a pKA of >7.4 (i.e. above physioloigic pH), and so when they are injected into normal tissue, a greater proportion are in the inactive ionised form. Generally, local anaesthetics with lower pKa values have a faster onset of action as more of the agent is in the non-ionised form (figure 3). For example bupivacaine has a pKa of 8.1 (~83% inactive non-ionised at pH 7.4), and lidocaine has a pKa of 7.7 (~76% inactive non-ionised at pH 7.4).
Local anaesthetics are often stored at an acidic pH, and virtually all of the drug will be in the inactive form. When the drug is injected there is a change in pH and this allows it to transform into the non-ionised active form. Likewise, when injected into an acidic environment due to infected or inflamed tissues, more of the anaesthetic will be in the inactive ionised form.
The intermediate chain is used to classify local anaesthetics into esters and amides. This determines the pattern of metabolism. Esters are hydrolysed in the plasma by esterases, while amides are metabolised in the liver (and kidneys). Both are excreted by the kidneys. Esters are not widely used, are poorly soluble, and have more side effects.
Local anaesthetics are also described by their protein binding tendency in plasma to alpha-1-acid glycoprotein. Increased plasma binding correlates with increased protein binding within sodium channels. Increased binding therefore increases the duration of action.
Other Considerations
Storage Considerations
Most of the amide anaesthetics are quite stable and don't require additives. When adrenaline is added, this has a destabilising effect, and so the anti-oxidant sodium metabisuphite is added in concentrations of 0.1%. This compound is an allergen in some individuals, and can also cause neurotoxicity in low pH settings. Care should be taken with spinal and epidural administration. Adrenaline reduces blood flow to the area and therefore reduces plasma concentration of the local anaesthetic and increases the duration of action. This effect is less with long acting anaesthetics.
Injection Considerations
The following factors act at the site of injection and play a role in local anaesthetic action
- Proximity to nervous tissue
- Rate of blood flow at the injection site
- Volume of injection
- Injection
Intravascular injection risk can be reduced by having a good anatomical knowledge, using shorter needles, aspirating before injection, and injecting slowly. Intravascular injection can still occur despite a negative aspiration. If concerned, remove the syringe while the needle is in place and observe for backflow.
Local anaesthetic maximum doses vary depending on the site of injection. For example intercostal nerve blocks have a much lower maximum safe dose. The onset of action also varies depending on the injection site. For example subarachnoid injection results in rapid blockade even with small volumes, while brachial plexus blocks have a slow onset of action even with large volumes.
The volume injected influences the duration of action. This is through increasing dispersion by bulk flow and diffusion
The total dose of anaesthetic is the main factor in efficacy, i.e. the concentration x volume. In peripheral nerve blocks, the outer fibres are blocked first (the mantle bundles), and subsequently the core fibres. In the limbs the proximal portion is supplied by the outer nerve fibres, and so the proximal portion will be blocked first and will also wear off first.
Toxicity
Central nervous system toxicity is determined by the absolute concentration and rate of absorption into the CNS. There is a stepwise increase in symptoms with increasing toxicity.
- Tongue numbness
- Lightheadedness, dizziness
- Visual and auditory disturbances
- Muscular twitching
- Unconsciousness
- Convulsions
- Coma
- Respiratory arrest
- Cardiovascular depression
Certain areas are highly absorbant. These include intercostal blocks, caudal and other epidural blocks, and major nerve trunk blocks. Subcutaneous infiltration is much slower.
The cardiovascular system is 4-7 times more resistant to local anaesthetics than the central nervous system. Therefore CNS side effects generally appear much earlier than cardiovascular effects. Bupivacaine has the highest cardiotoxicity
The treatment of CNS toxicity is largely supportive, with the use of lipid emulsion is severe cases. See Life in the Fast Lane for detailed management.
Allergy
Patients can be allergic to the preservative methylparaben or metabisulphite. Allergic reactions are more common with ester anaesthetics.
Local Anaesthetic Resistance
Dr Trescott tested 250 patients with a history of anaesthetic failure in their response to mepivicaine, lidocaine, and bupivacaine. 7.5% were found to respond only to mepivicaine, and an additional 3.8% only to lidocaine. The rest were responsive to all three anaesthetics or bupivacaine. She concluded that patients are questioned on any history of poor response to local anaesthetic infiltration (e.g. at the dentist), and are skin tested prior to any invasive procedure.[3]
Patients with Ehlers Danlos Syndrome may be more susceptible to local anaesthetic resistance, however the evidence for this association is limited to case series and a survey.[4]
There has been some genetic studies on this topic, and candidate genetic variants of voltage gated sodium channels have been identified.[5]
The concept of local anaesthetic resistance is not widely supported or appreciated in New Zealand, or it is thought to be extremely rare. There are some factors that may explain failure apart from pure resistance that should be considered.
Local anaesthetics work the quickest on small nerve fibres. After blockade of these smaller fibres, the larger pressure sensing fibres will still be active. It may be that some patients are actually sensing pressure rather than pain, and due to the anxiety of having a procedure done are interpreting pressure as pain. In these patients it is anecdotally often helpful to give the anaesthetic a much longer time to work to enable blockade of these larger fibres.
Applications
In Musculoskeletal Medicine local anaesthetics are used for
- Diagnostic blocks of peripheral nerves or spinal nerves
- Co-administered with a separate agent for therapeutic effect
- In trigger point injections
Resources
- NYSORA for an open access review on the pharmacology of local anaesthetics, pending article creation.
- Becker et al
References
- ↑ Cramariuc O, Rog T, Javanainen M, Monticelli L, Polishchuk AV, Vattulainen I. Mechanism for translocation of fluoroquinolones across lipid membranes. Biochim Biophys Acta. 2012 Nov;1818(11):2563-71. doi: 10.1016/j.bbamem.2012.05.027. Epub 2012 Jun 1. PMID: 22664062.
- ↑ Factors which determine the lipid solubility of drugs. Deranged Physiology. Text
- ↑ Trescot. Local anesthetic "resistance". Pain physician 2003. 6:291-3. PMID: 16880874.
- ↑ Schubart et al.. Resistance to local anesthesia in people with the Ehlers-Danlos Syndromes presenting for dental surgery. Journal of dental anesthesia and pain medicine 2019. 19:261-270. PMID: 31723666. DOI. Full Text.
- ↑ Clendenen et al.. Whole-exome sequencing of a family with local anesthetic resistance. Minerva anestesiologica 2016. 82:1089-1097. PMID: 27243970.