Nerve Conduction Studies
Nerve conduction studies (NCS) allow the stimulation and recording of peripheral nerve function. Electrical stimulation causes an impulse that travels along motor, sensory, or mixed nerves. Potentials are recorded from either the muscle innervated by the motor nerve, or from the nerve itself. NCS is used to diagnose focal and generalised peripheral nerve disorders; aid in the differentiation between primary muscle and nerve disorders; classify peripheral nerve conduction abnormalities as being due to demyelination, axonal degeneration, or conduction block; and provide a prognosis on treatment effect and clinical course.
Anatomy and Normal Neurophysiology
Most peripheral nerves are mixed, comprising motor, sensory, and autonomic functions. There are certain primarily motor (e.g. anterior interosseous branch of the median nerve, posterior interosseous branch of the radial nerve) and primarily sensory fibres (e.g. sural nerve, peripheral peroneal nerve, superficial radial nerves) that allow neurophysiologic investigation.
Individual nerves are generally classified based on their diameters, conductivity, and myelin properties.
- A-alpha (or A-alpha beta): large myelinated fibers, 6 to 15 microns in diameter. The largest muscle afferent fibers are sometimes classified as 1a fibers.
- A-delta: small myelinated fibers, 3 to 5 microns in diameter.
- C fibers: unmyelinated fibers, 0.5 to 2 microns in diameter.
Most efferent motor fibres are A-alpha (large myelinated). A-alpha fibres also include sensory fibres providing touch, vibration, and position senses. Cold and fast pain sensations are mediated by A-delta fibres (small myelinated). Warm, itch, and slow pain sensations are mediated by C fibres (unmyelinated). C fibres also include efferent postganglionic sympathetic autonomic fibres.
Small fibre neuropathy - where strength, vibration, and position are normal, but pain and temperature are abnormal - poses a problem with nerve conduction studies. This is because NCS can only evaluate the largest A-alpha fibres. In other words, NCS is normal in pure small-fibre neuropathies. A skin biopsy may be required for diagnosis in this case.
Compound action potentials
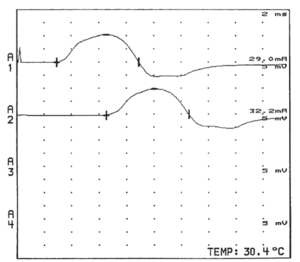
A-alpha fibres generate sensory nerve action potentials (SNAPs) and compound muscle action potentials (CMAPs). SNAPs and CMAPs are compound potentials representing summated electrical activity of individual nerve fibres with simultaneous activation of nerve stimulation. A-delta fibre electrical activity can be visualised through special techniques.
Myelinated fibre nerve conductivity is saltatory, via Ranvier (node-to-node) depolarisation. The speed of conduction is dependent on the nerve fibre diameter, with the largest fibres conducting the fastest. The relationship between diameter and velocity is roughly linear at a conversion factor of around 4.3m/s/micron in healthy individuals. 14 to 15 micron A-alpha fibres have a conduction velocity of around 65 to 70 m/s, and 6 to 7 micron A-alpha fibres at around 30 to 35 m/s
The SNAP peak-to-peak amplitude and area under the waveform represents the number of activated nerve fibres. The CMAP amplitude relates more to the number of activated muscle fibres rather than nerve fibres.
With an acute nerve lesion, before the onset of nerve regeneration, there is a loss of motor nerve fibres in parallel with the loss of activatable muscle fibres. Therefore CMAP amplitude is reduced. With chronic lesions, surviving motor units reinnervate the previously denervated muscle fibres through sprouting of collateral terminal motor nerve fibre branches. There are subsequently reduced numbers of motor fibres, each having increased motor unit territories and increased muscle fibre per motor units numbers. Therefore in this situation, CMAP amplitude is a poor estimate of motor nerve fibre number. The CMAP can have a normal or only mildly reduced amplitude. Motor unit number estimation (MUNE) and electromyography can be helpful in this situation.
Temporal dispersion
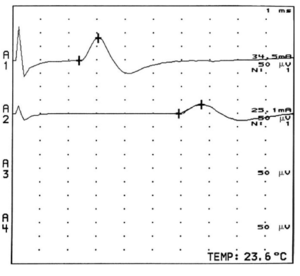
The largest and fastest conducting A-alpha fibres contribute to the earliest part of the compound evoked potentials, while slower conducting fibres contribute to the later parts. This is called temporal dispersion of electrical activities. The onset latency of a compound evoked potential is determined by the conduction speed of the fastest fibres. The duration of the evoked potential can be measured from the time it leaves the baseline to the time when it returns to the baseline.
An analogy for temporal dispersion is a race between a Greyhound and a Dachshund over a distance of 1 kilometre. The greyhound can run at a speed of 70km/hr, while the Dachshund can run at 30km/hr. Shortly after the start of the race, the dogs are bunched together. The dog "concentration" is at it's highest level, and this represents the CMAP amplitude. Also at the start, the spread between the Greyhound and Dachshund is shortest. As the race progresses, the dogs spread out, and so their concentration at any one point decreases. When the Greyhound reaches the finish line, the Dachshund is 1 minute and 10 seconds behind. At the finish line the concentration (amplitude) of dogs is less than previous points in the race, and the distance (duration) between the Greyhound and Dachshund is the greatest. In other words, with nerve conduction, the amplitude is greatest at the beginning, lessening as time goes on. This reflects the differences in conduction speed of the different A-alpha fibres. So when the distance increases between the stimulating and recording electrodes, you get an increase in temporal dispersion, and a decrease in amplitude.
Temporal dispersion can be characterised as abnormal with an an excessive difference in slowing between individual motor axons within a nerve. This is generally due to altered or damaged myelin. There are different criteria defining abnormal temporal dispersion. For example the American Association of Neuromuscular and Electrodiagnostic Medicine define abnormal temporal dispersion as a 30 percent or more increase in CMAP duration between proximal and distal sites. Note that the relationship between increasing nerve length segment and temporal dispersion is roughly linear for motor conduction, but not for sensory conduction.
Phase Cancellation
The recorded potentials of both single motor units and single sensory fibres have both positive and negative components. Therefore phase cancellation can occur depending on the conduction velocity of the individual fibres. Phase cancellation is more significant with increasing distances between the stimulating and recording electrodes.
Individual sensory nerve fibres contribute differently to SNAPs, and individual motor nerve fibres contribute differently to CMAPs. A single motor unit potential has a much longer action potential than a single large myelinated sensory nerve fibre (5-15msec vs 2msec). With the shorter sensory nerve fibre potential, small amounts of dispersion can cause a dramatic reduction in SNAP amplitude compared to CMAP amplitude. Increasing the conduction distances decreases median and ulnar SNAP amplitudes by 16-17 times more than the corresponding CMAP amplitude decrease. The extent of SNAP amplitude reduction with increasing distance is not defined by any criteria. The distance is important when considering the diagnostic criteria for demyelinating nerve diseases.
Saltatory Conduction
Nerve Conduction Physiologic Factors
Methodology
Abnormal Findings
Clinical Applications
Key Points
See Also
References
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,


