Internal Disc Disruption: Difference between revisions
No edit summary |
mNo edit summary |
||
| Line 64: | Line 64: | ||
== References == | == References == | ||
{{Reliable sources}} | {{Reliable sources}} | ||
[[Category:Lumbar Spine]] | <references /> | ||
[[Category:Lumbar Spine Conditions]] | |||
Revision as of 09:11, 7 September 2021
Internal disc disruption is a condition that affects lumbar intervertebral discs and is the cause of pain in around 40% of individuals with chronic low back pain. It is defined as the presence of isolated, radial fissures that penetrate from the nucleus pulposus through to the anulus fibrosus, however the outer anulus is not breached.[1]
It is a separate concept to disc degeneration. While there are many similar processes and features, internal disc disruption is a response to injury that occurs either through single or repetitive compressive loads.
Disc Innervation
Nerve endings are present in the outer one third of the annulus fibrosus. There are nerve endings in the inner annulus. The posterior aspect it supplied by the sinuvertebral nerve. The anterolateral aspect receives branches from the grey rami communicantes of the lumbar sympathetic trunk.
“Some fibers innervating the anterior and lateral aspects of the discs, and to a lesser extent even the posterior aspect of the IVDs, have been found in animal models (rats) to ascend several segments in the sympathetic chain before passing through a gray communicating ramus, anterior primary division, and dorsal root and ganglion to enter the dorsal horn of the spinal cord several segments higher than the level of innervation. This is thought to explain the broad referral patterns (e.g., pain referred into the inguinal region from IVD pathology as low as L5-S1) seen in various presentations of low back pain of primary discal origin”
—Cramer G, Darby S. Clinical Anatomy of the Spine, Spinal Cord, and ANS - E-Book. Chapter 7.Elsevier Health Sciences 2017. Retrieved from https://books.google.co.nz/books?id=9upfXATNzjgC
Aetiology
End-plate fracture
Internal disc disruption occurs following injury to the overlying vertebral end plate. This can occur following a single sudden compressive force, or through fatigue failure from repetitive lower forces. End plate fracture can occur with as few as 100 repetitions with compressive loads as low as 50% to 60% of the ultimate tensile strength of the end plate.[2] Torsional tears to the disc can result from forcible rotation about the facet joint, with the disc most susceptible in flexion-rotation.[3]
Once end-plate fracture has occurred, disc degradation can occur. While the exact sequence of events is uncertain, it is well established that end-plate fracture is an important inciting event. Mechanistic theories include an inflammatory reaction to proteins of the nucleus, and a reduction in the pH leading to an increased activity of metalloproteases.
Following matrix degradation, it is thought that the matrix isn't able to brace the anulus from buckling inwards. It may be that continued normal activities of every day life progressively leads to tearing of the anulus in this unsupported state with increased loads placed on it.
Biomechanics
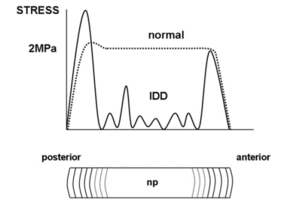
Biomechanically there are significant changes in the internal stresses. In the normal state, while the outer portion of the anulus has no compression stresses, the inner portion of the anulus and the nucleus pulposus have uniform stresses, with a small peak in the posterior anulus.
In the setting of internal disc disruption, the disc is not able to bear compression loads normally. There are irregular stresses that have reduced amplitudes, even reaching zero in some areas. This pattern is seen immediately following end-plate fracture.
Correlation with Pain
Radial fissures aren't degenerative or age related changes as they are independent of these two factors. They are strongly associated with the disc being painful on provocative discography.
Central sensitisation or "primary pain" is not the typical cause of pain. This has been shown in studies where discography has been done in adjacent segments finding non-painful discs. If these patients had central sensitisation then all discs would be painful, but this is not what is found. Patients with elevated levels of psychological distress may have elevated pain scores with discography, but the association is not absolute. Furthermore, spatial summation as is seen in central sensitisation does not in low back pain.[5]
The pain is thought to be primarily nociceptive. Nociception is thought to occur through interaction of nuclear material with the outer third of the annulus, which is where nociceptive fibres are located, which is why grade III and IV fissures are more likely to be painful. Both chemical and mechanical nociception is theorised to occur.
Chemical nociception occurs via degraded nucleus pulposus matrix and inflammatory chemicals. NGF expression correlates with microvascular and nerve fibre ingrowth into the annulus fibrosus and nucleus pulposus.[6] TNF-a has been shown to provoke biochemical degenerative changes in vitro, and possibly mediates pain (as does IL-1b).[7] Mechanical nociception is thought to occur via radial fissuring leading to increased loading on the remaining intact lamellae
Neoinnervation (sympathetic and nociceptive fibres) also occurs along radial fissures, and this process can lead to innervation of the internal anulus and even the nucleus.
Classification
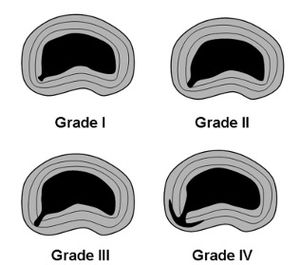
Fissures can be graded based on the extent of penetration into the anulus. The outer anulus can bulge over a fissure but it remains intact, i.e. there is no penetrating defect externally.
- Grade I: reaches the inner third of the anulus
- Grade II: reaches the middle third of the anulus
- Grade III: reaches the outer third of the anulus
- Grade IV: the fissure spreads circumferentially around the annulus
Grade III and IV fissures are most likely to be painful. It is uncommon for discs without any fissure or only grade I or II fissures to be painful.
Epidemiology
Internal disc disruption is common in those with chronic low back pain who have invasive tests, with different studies reporting prevalence rates of 39%, 26%, and 42% in patients attending specialist clinics using stringent diagnostic criteria: positive discography and a grade 3+ fissure on post-discography CT.
Clinical Features
There may be pain at rest which is thought to arise from chemical nociception, and pain on movement which is thought to arise from mechanical nociception. There are no neurological features. In other words it presents just like any typical low back pain
Investigations and Diagnosis
The diagnosis of internal disc disruption can be made through post-discography CT scan imaging. With discography, contrast is injected into the nucleus. With the contrast in place, CT imaging shows up any fissures and their grade. post-discography CT is not commonly done in the clinical setting.
MRI can be used to look for two specific findings: modic changes and high intensity zones. Both of these features are independently correlated with pain, however they are not exclusive to the symptomatic population. They are not pathognomic for that segment being the cause of pain, but are highly specific.
- Modic changes occur around the endplates and represent current (modic type I) or past (modic type II) inflammatory changes. Sensitivity is 24%, specificity 83%, and positive likelihood ratio 3.4.[1]
- High intensity zones represent circumferential annular tears. Sensitivity is 45%, specificity 88%, and positive likelihood ratio 3.8.[1]
For determining whether a particular disc is painful, the gold standard is provocative discography. This is not commonly done in New Zealand, despite a false-positive rate of only 10%. MRI findings are used as a substitute, as their presence in the context of low back pain constitutes a 70% post-test probability of the affected disc being painful based on a pre-test probability of 40%.[1]
Philosophically, diagnosis is important for its negative therapeutic utility (it makes certain interventions less likely to be recommended such as surgery in the context of negative or indeterminate discography), and diagnostic utility (it provides an explanation and stops the search for a diagnosis). There does not appear to be a good argument for its positive therapeutic utility (whether diagnosis leads to a better outcome) due to lack of high-quality research looking at this question.[1]
References
Literature Review
- Reviews from the last 7 years: review articles, free review articles, systematic reviews, meta-analyses, NCBI Bookshelf
- Articles from all years: PubMed search, Google Scholar search.
- TRIP Database: clinical publications about evidence-based medicine.
- Other Wikis: Radiopaedia, Wikipedia Search, Wikipedia I Feel Lucky, Orthobullets,
- ↑ 1.0 1.1 1.2 1.3 1.4 Bogduk N, Aprill C, Derby R. Lumbar discogenic pain: state-of-the-art review. Pain Med. 2013 Jun;14(6):813-36. doi: 10.1111/pme.12082. Epub 2013 Apr 8. PMID: 23566298.
- ↑ Hansson TH, Keller TS, Spengler DM. Mechanical behaviour of the human lumbar spine. II. Fatigue strength during dynamic compressive loading. J Orthop Res 1987;5:479–87
- ↑ Farfan HF et al. The effects of torsion on the lumbar intervertebral joints: the role of torsion in the production of disc degeneration. J Bone Joint Surg Am. 1970;52(3):468–97
- ↑ Bogduk N. Degenerative joint disease of the spine. Radiol Clin N Am 2012;50(4):613–628
- ↑ Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005 Mar;114(1-2):295-302. doi: 10.1016/j.pain.2004.12.032. PMID: 15733656.
- ↑ Freemont A et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286–92
- ↑ Séguin CA et al. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue.Spine 2005.30(17): 1940–8
- ↑ Bogduk N. Degenerative joint disease of the spine. Radiol Clin North Am. 2012 Jul;50(4):613-28. doi: 10.1016/j.rcl.2012.04.012. PMID: 22643388.


